Carboxylic Acid Reactions: Fischer Esterification using Carboxylic acids and Alcohols
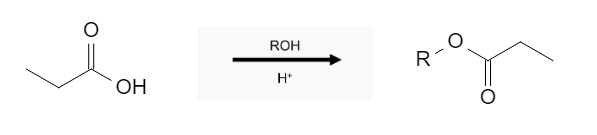
Carboxylic acids and alcohols in an acidic environment react to form esters:
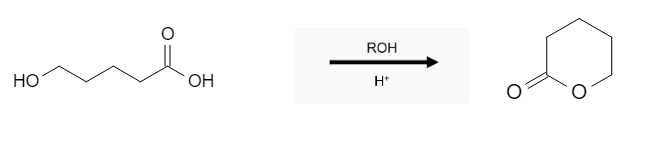
This reaction is pretty straight forward; the OH group gets replaced with an ROH group; that could be methanol (MeOH) or even something a little larger like cyclohexanol. If the molecule itself has an alcohol group, an intramolecular reaction will occur forming a cyclic ester:
Intramolecular Reaction
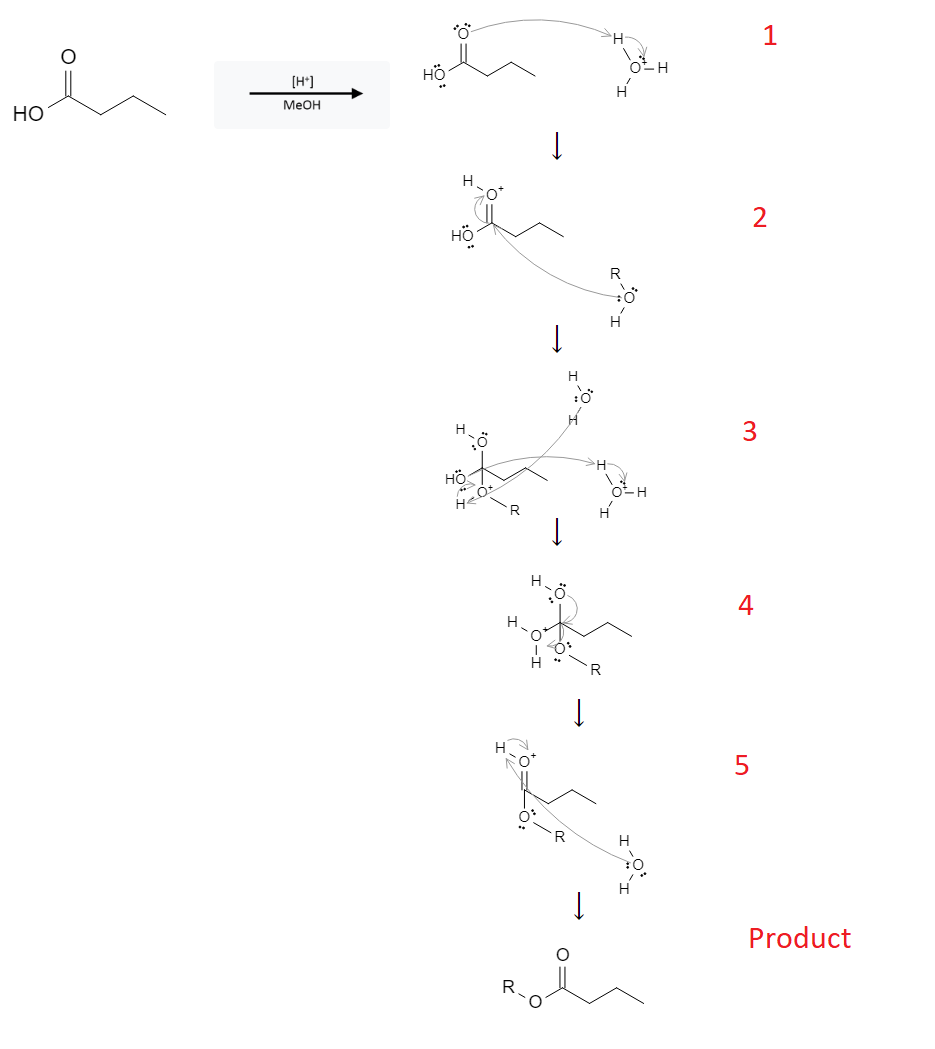
The reaction mechanism is depicted below:
In the first step, the free electrons from the double bonded oxygen atom remove a proton from a nearby H3O+ molecule.
In the second step, a nearby alcohol molecule (MeOH, ROH) attacks the carboxylic acid-carbon, breaking the double bond with the oxygen sending those electrons to the oxygen atom.
In the third step, the lone pair electrons from the carboxylic acid-oxygen atom attack a nearby proton on an H3O molecule while simultaneously, an H2O molecule removes a free proton from the newly bonded ROH molecule.
In the fourth step, the OH groups attack the carboxylic acid-carbon, forcing the recently protonated oxygen off the original molecule.
In the fifth step, a nearby water molecule strips the proton from the double bonded oxygen atom, completing the reaction.
This reaction is conducted using any alcohol (ROH) in an acidic environment (AKA H+ or ‘acid workup’).