The Organic Chemistry of Ibuprofen
If you’ve ever been sick and had a fever, you might have taken ibuprofen (Advil) to help control your fever and reduce any pain or inflammation. But have you ever wondered how it’s made? Or what's inside it? As organic chemistry students, you have the fundamentals to understand the chemistry and pharmacology behind how one of the most common over the counter analgesics are made!
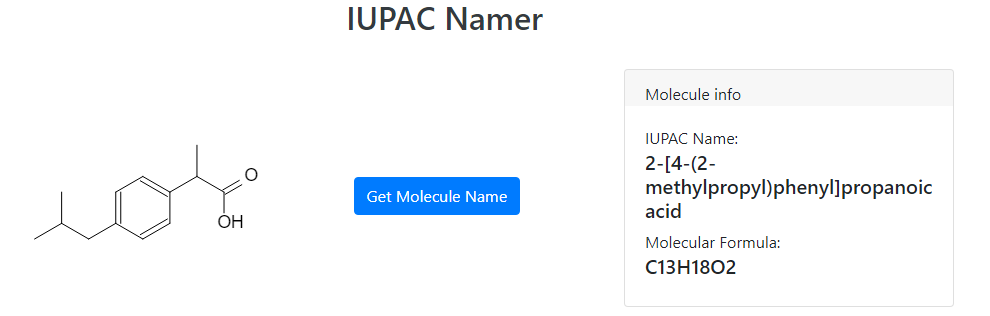
Let's start at the end and see what ibuprofen looks like in the first place:
Ibuprofen’s IUPAC name is actually 2-[4-(2-methylpropyl)phenyl]propanoic acid, what a mouthful! I’m glad we just call it ibuprofen instead.
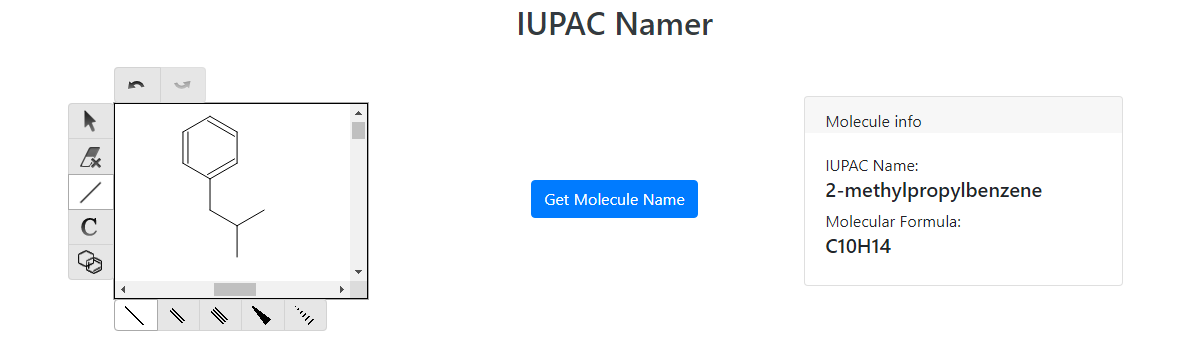
To synthesize ibuprofen, start with 2-methylpropyl benzene:
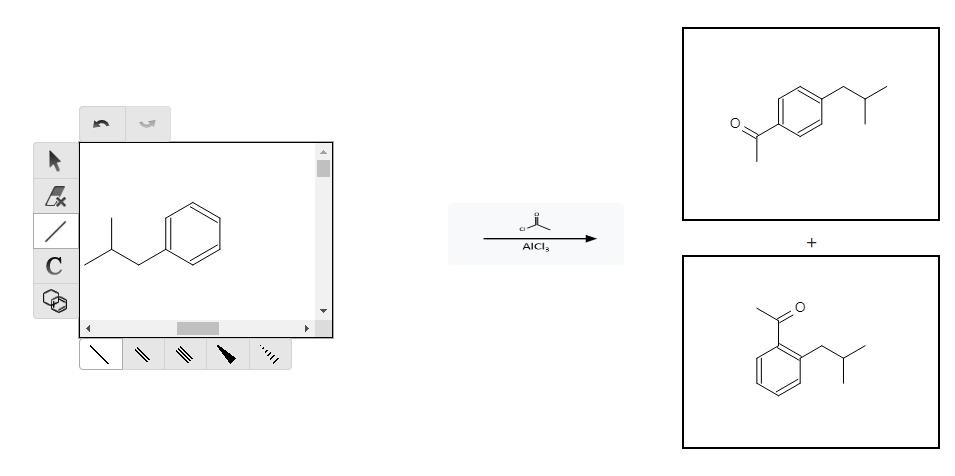
As you can see, with this starting reactant we already have the methylpropyl group attached. Now we have to figure out how to add the other chain to the molecule. A common reaction to add a carbon chain to a benzene molecule is the Friedel-Crafts Acylation:
We can see that there are two products here, one where the acylation occurs at the ortho position and one where it occurs at the para position. Because our product needs to be at the para position, we are already losing potential yield from our reaction! This is built into the production of the drug.
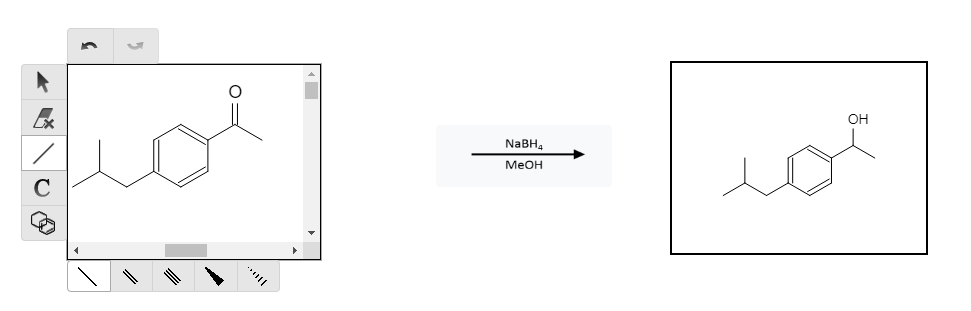
We are close to our desired result, but we still need to add some carbons and “move” the ketone group and convert it to a carboxylic acid group. The next step would be to reduce the ketone group to alcohol so that we can further react the alcohol group. We do that by performing a reduction with NaBH4 and methanol (MeOH):
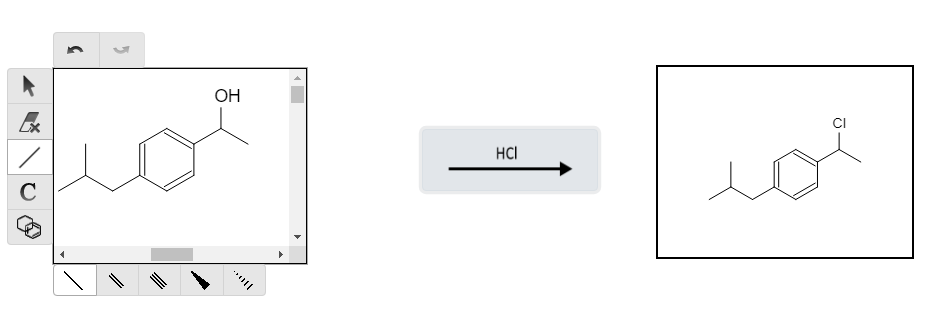
Getting closer! If this were an organic chemistry exam synthesis problem, my advice would be to look for which groups can still react. Right now, the OH group is the only group that would likely react with any reagent. We can perform a hydrochlorination to replace the OH with a Cl group that will enable us to have a more reactive leaving group:
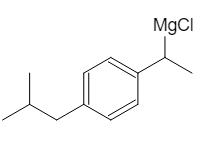
Now that we have substituted Cl for OH, we can use magnesium (Mg) and add it to the mixture. Since we have a Cl group, the Mg will react with that group to form a Grignard reagent:
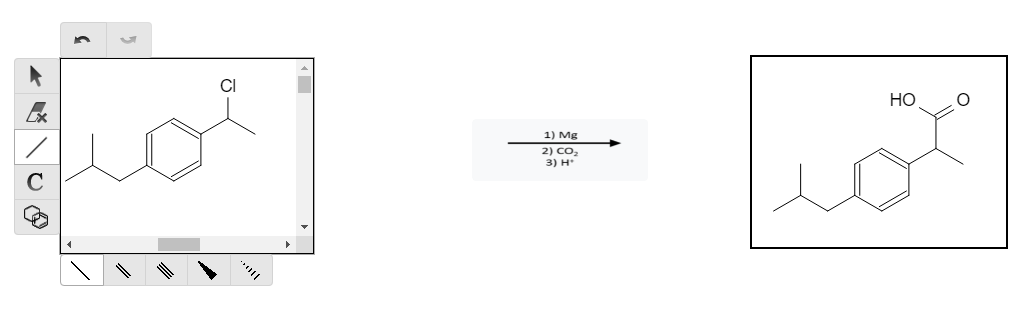
Grignard reagents are very reactive since MgCl is an excellent leaving group. A common organic chemistry reaction is to react a Grignard reagent with CO2 in an acidic environment (H+, H3O+, HCl) to “add” CO2 to the molecule in the form of a carboxylic acid group:
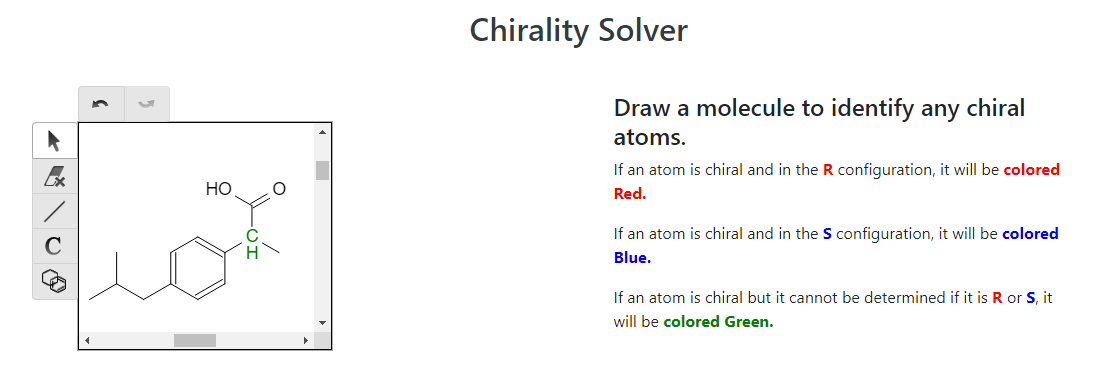
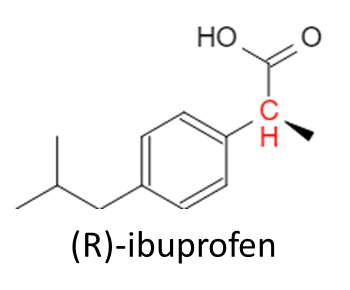
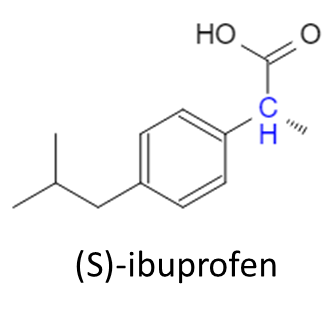
And that completes the synthesis of ibuprofen! But, there’s more to the story. The effectiveness of ibuprofen is determined by the stereochemistry of the enantiomer. Looking at the ibuprofen molecule above, can you determine where the stereocenter is? Let’s use OrgoSolvers Chirality Solver to see if there are any stereocenters:
Uh oh, the carbon group that we added on has a chiral center. This means that, depending on the 3D geometry of the synthesis reactions that took place, there are 2 enantiomers that can be synthesized: (R)-ibuprofen and (S)-ibuprofen. (For more information on chirality and stereochemistry, check out our chirality lesson)
So what’s the big deal? Surely it’s the same molecule with 1 small difference, that wouldn’t mean very much to the human body would it? Well, why would I be writing this if it didn’t! Amazingly, this small difference that occurs spontaneously during the reaction process confers benefits to the (S) enantiomer, while making the (R) enantiomer almost useless. Ibuprofen works by inhibiting an enzyme in our body (cyclooxygenase or COX) which converts arachidonic acid to prostaglandin H2, which is a precursor to numerous other signaling molecules (like ones that cause pain, inflammation, and fever). When you take ibuprofen, you block this reaction and therefore, these signaling molecules are also not synthesized. This is what gives us the perception of pain reduction and fever reduction; our body would otherwise be signaling that something is wrong but we “block the call” using ibuprofen, so these signals are never created. The (S) enantiomer is said to be 10x more potent than the (R) enantiomer. This is explained in biochemistry through inhibition and the general affinities enzymes have for certain molecules (the COX enzyme has a higher affinity for the (S) enantiomer over the (R)). This means that when ibuprofen is synthesized in a lab, about 50% will be (S) and 50% will be (R) but, the therapeutic benefit is entirely from the (S) enantiomer. You might be thinking, “well, why don’t we specifically synthesize just the (S) enantiomer?” and actually we do make it! (S)-ibuprofen is the main active ingredient in a drug called Seractil! But as you can imagine, it costs substantially more than generic OTC ibuprofen. Since there aren’t any specific drawbacks to the (R) enantiomer, ibuprofen is sold with the (S) enantiomer forming a racemic mixture which is then packaged into pills or tablets.
And there you have it! Real world applications of the reactions and stereochemistry principles taught in organic chemistry!
Good luck in your studies!