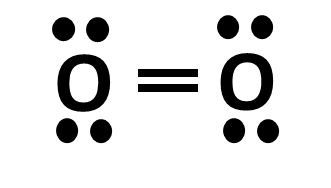Bond Types and Orbitals
In organic chemistry, almost every bond encountered will be a covalent bond. A covalent bond means that electrons are shared between the 2 atoms. This is in contrast to an ionic bond where one atom is much more electronegative than the other atom and often takes all of the electrons when the bond is broken. There are 3 bonds you will see in organic chemistry: single bond, double bond, and triple bond.
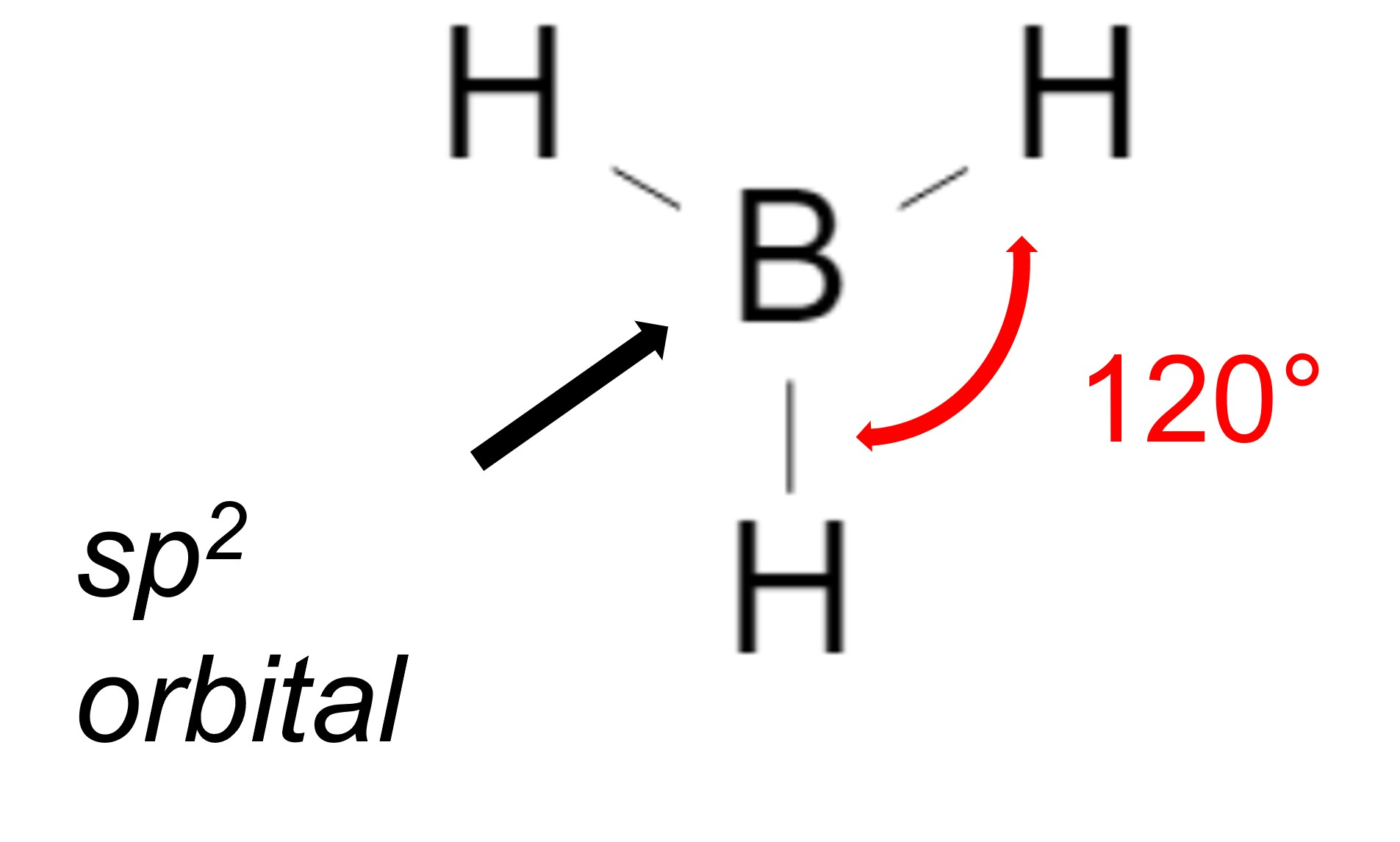
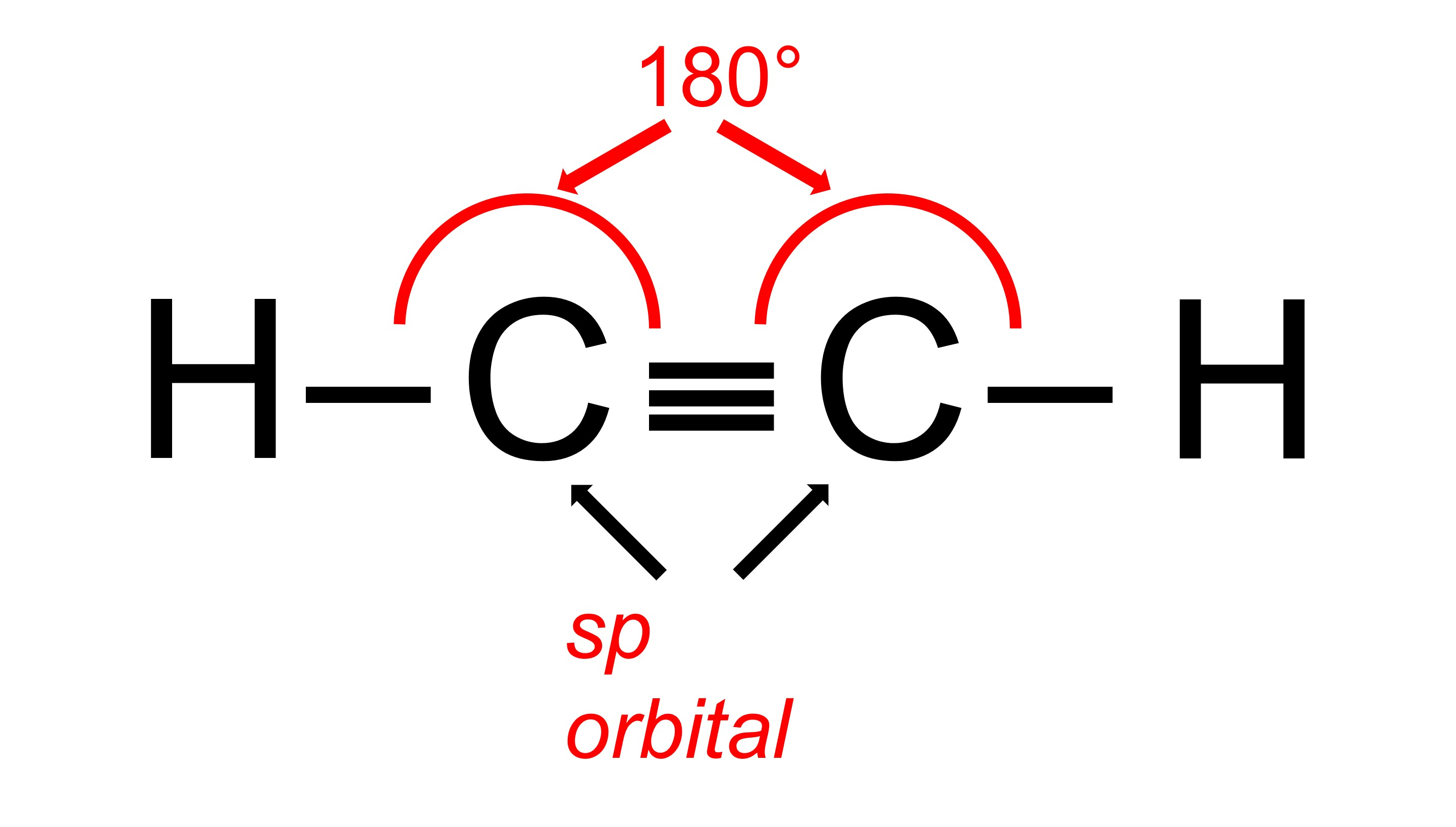
Just as there are 3 types of bonds, there are 3 types of orbital hybridizations: sp, sp2, and sp3:
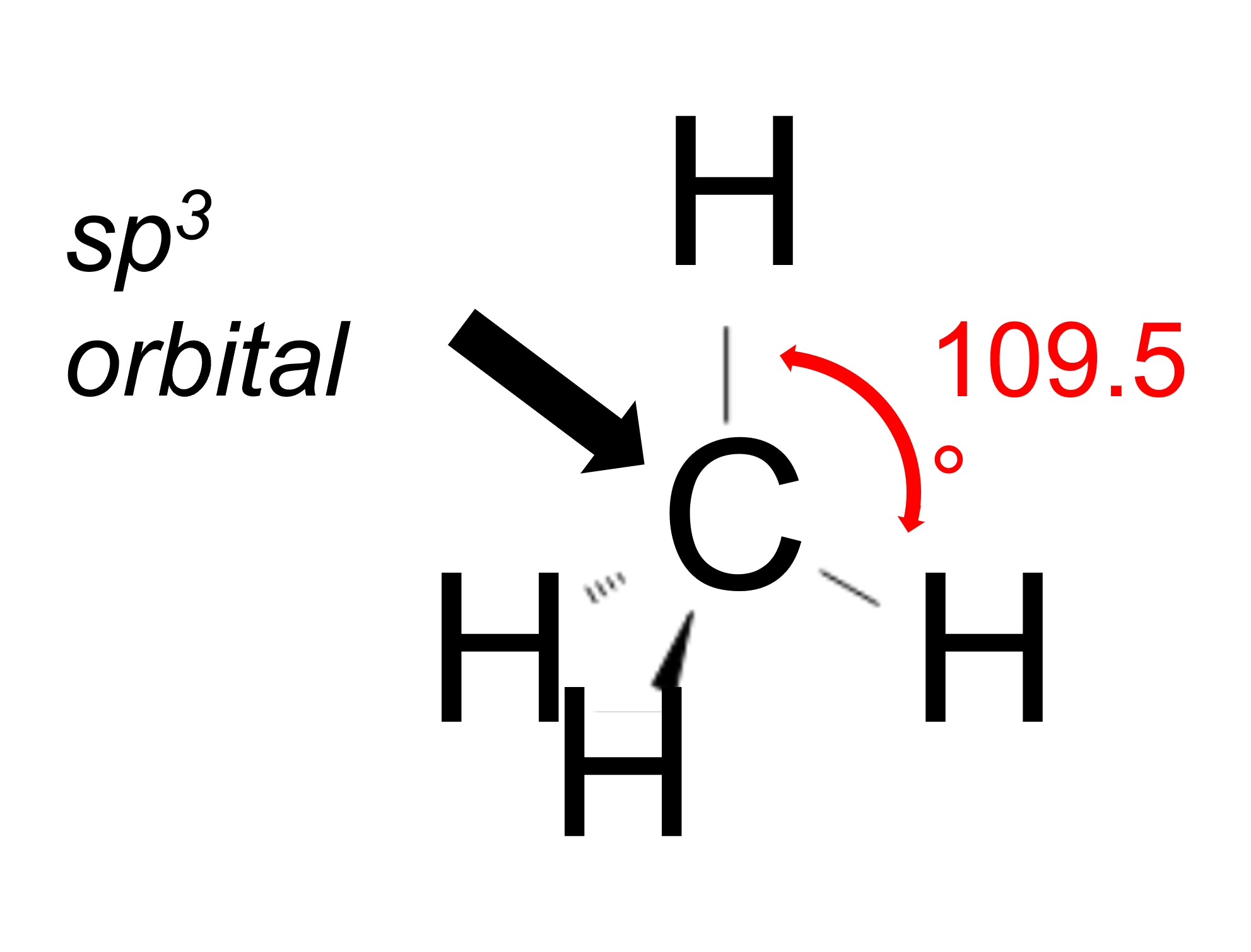
Each orbital hybridization describes the bond angles for that atom. In an sp3 hybridization, there are 4 bonds and each attached atom wants to be as far as possible from every other attached atom. In this case, the bond angle is close to 109.5 degrees; if you imagine the molecule in 3D, the best way to get every atom separate from each other one is to have one atom coming towards you, one towards the top of the molecule, one going to the back right and one going to the back left. This creates a pyramid or tetrahedral shape.
In an sp2 hybridization, there is usually at least one double bond involved, which means that there will be 3 total bonds. The maximum distance each bonded atom can be from one another is 120 degrees. Finally, in an sp hybridization, there is usually a triple bond which means there will be 2 total bonds. The maximum distance each bonded atom can be from one another would be 180 degrees.
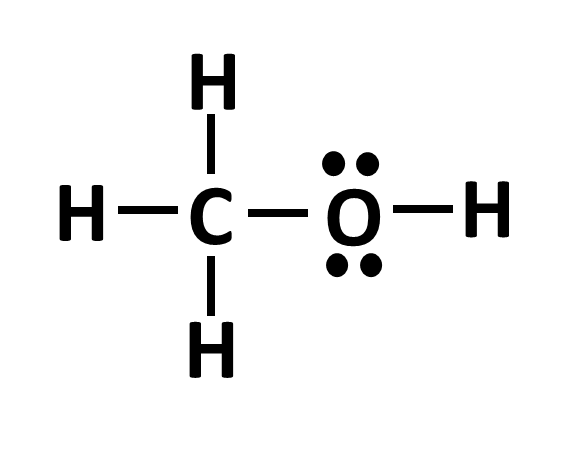
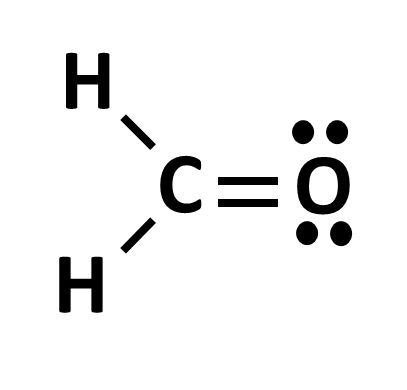
While bonds are important in determining the hybridization, lone pair electrons also play a role. When determining an atom’s hybridization that has lone pair electrons, treat the lone pair as if it was a single bond.
Take the two examples above. On the left, we see an O with 2 single bonds and 2 lone pair electrons. Treating the lone pairs as single bonds, we assign the O an sp3 orbital and the distance between each electron pair and each bond will be 109.5 degrees. On the right, the O has a double bond, but still has its 2 electron pairs. We would therefore assign this oxygen an sp2 hybridization as there is more room to spread out.
Polar Bonds and Dipole Moments
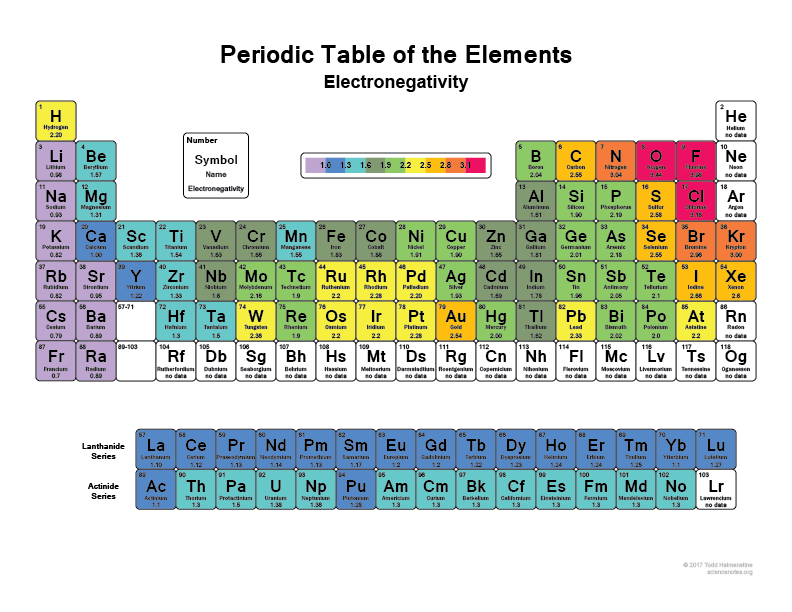
Different atoms have different levels of electronegativity. The level of electronegativity is an implicit property of the atom, that is, the electronegativity of an atom is determined by its location on the periodic table. You may have seen a periodic table similar to the one below that illustrates the electronegativity of all atoms:
Here we see that F is more negative than O, which is more negative than N, which is more negative than C, and finally H which is the least electronegative.
If we draw a molecule with a bond between C and O, we would expect the electrons to be pulled closer to the more electronegative molecule (in this case the O molecule). We denote this by drawing a lower case delta or δ:
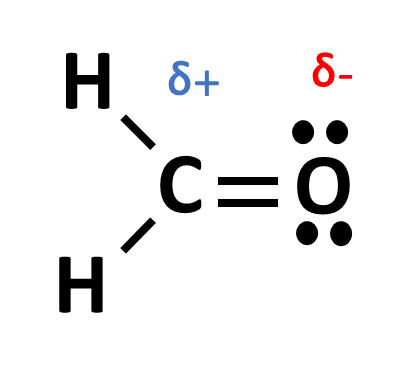
In this example, the O is more electronegative than C so we denote that by drawing δ with a - symbol on the O side and a δ with a + symbols on the C side.
Hydrogen Bonds
Lone pair electrons are especially electronegative and have the ability to pull atoms from other molecules. Hydrogens are not electronegative and can be easily influenced by lone pair electrons. Let's take a look at the example below:
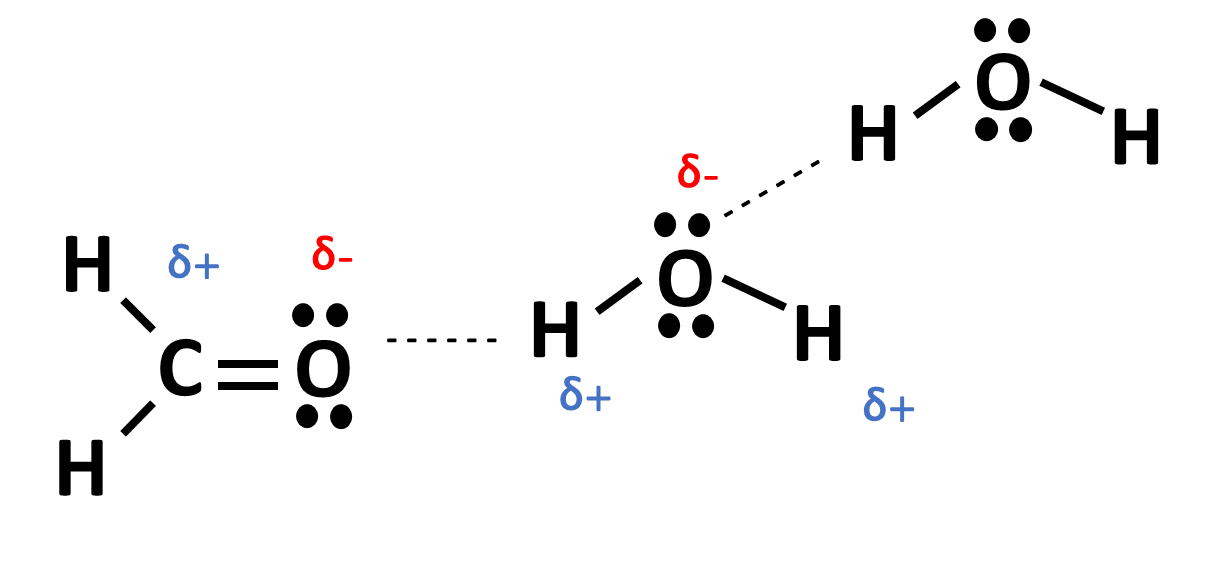
In this example, we see the lone pair from formaldehyde molecule (CH2O) is attracting the H from water (H2O). This happens frequently in polar solutions and it is known as a hydrogen bond. The dashed line signifies that, while no actual bond is being formed, there is a strong attraction between those two atoms. This is possible because simultaneously, there is another water molecule whose H atom is being attracted away by the O atom on the original water molecule. This fluid movement of electrons and attraction provides solutions with unique properties; hydrogen bonding is one reason why the boiling point of water is so much higher than would be scientifically expected. Hydrogen bonds also provide stability to molecules in solution and play a big role in acid-base chemistry. Hydrogen bonding and polarity are also important concepts when considering the solubility of two molecules with one another; the phrase “like dissolves like” refers to polar solutions that are able to mix with one another.
Citations
- Helmenstine, Todd, et al. “List of Electronegativity Values of the Elements.” Science Notes and Projects, 2 May 2021, https://sciencenotes.org/list-of-electronegativity-values-of-the-elements/.