Resonance Structures
Try our Resonance Solver Study Tool
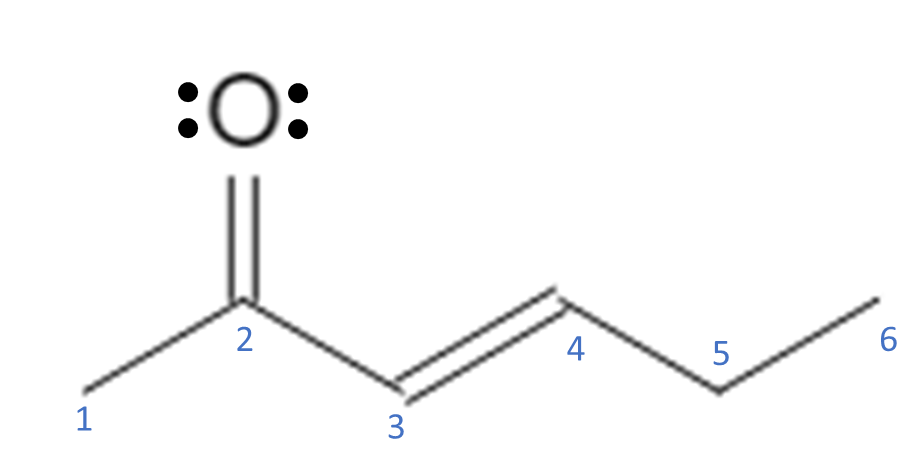
Moving electrons around is a fundamental part of organic chemistry. When we draw a molecule using bond-line structures, we see that there is typically a carbon backbone comprised of single bonds with some double bonds and other atoms (like O and N) mixed in:
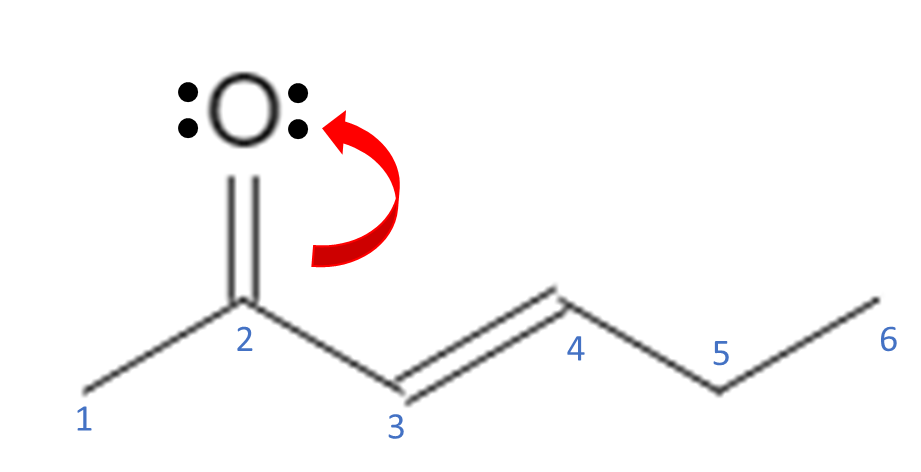
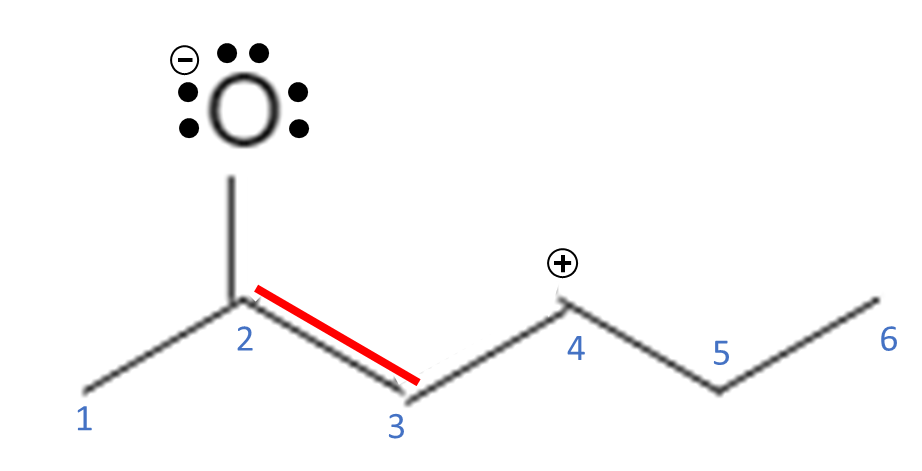
Double bonds, triple bonds, and lone electron pairs are sources of free electrons that are capable of moving around the molecule; single bonds are not usually broken. Free electrons can move around to stabilize the molecule by delocalizing the electrons and spreading them out across the molecule. In the example above, we have a double bond between C3-C4 and another double bond between a C2-O. O also has 2 lone pairs of electrons. For electrons to move around, they must be adjacent (next to) another atom that can accept them. For this molecule, let’s move the electrons from the O double bond up to the O, giving the O 3 lone pairs of electrons and 1 bond.
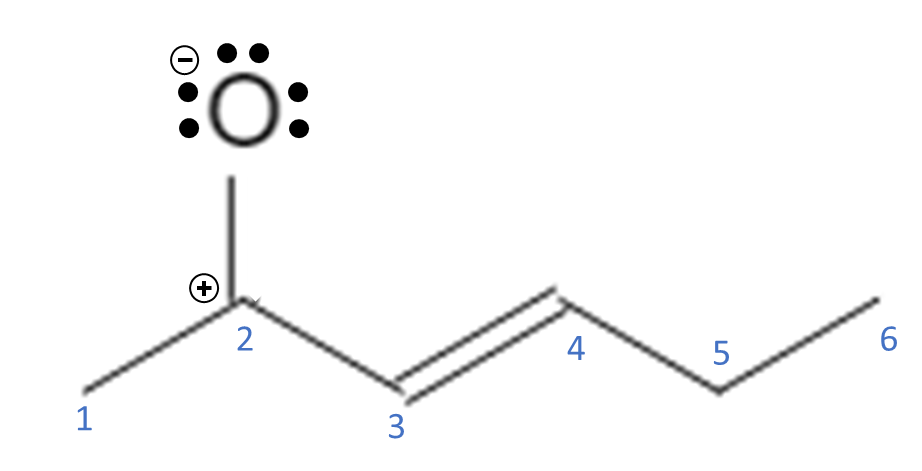
This makes the O have a full octet but it now has 7 total electrons in its valence shell, so now it has a negative charge. Since we donated the electrons from the double bond to the O, the C now only has 3 bonds and no lone pairs. C now has 6 electrons associated with it but it has 3 electrons in its valence shell, so now it has a positive charge.
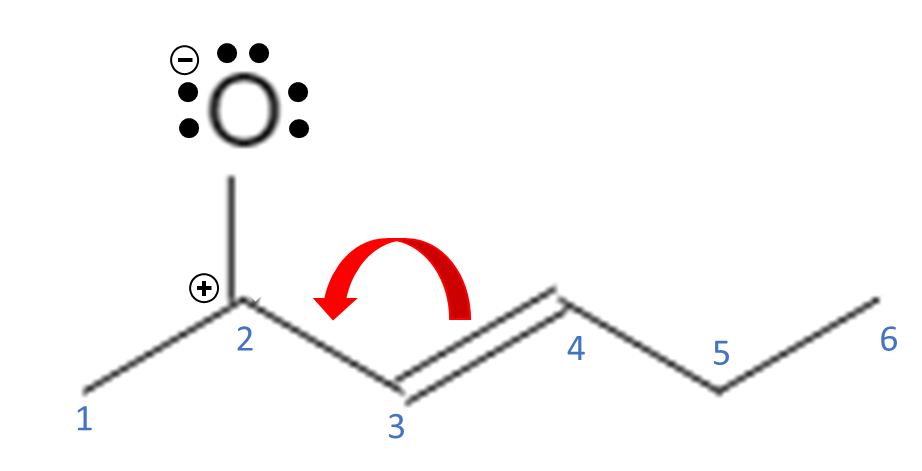
The positive charge causes C2 to be an electron acceptor. It could bond with the O if it donates the 2 electrons that it just took, but it can also form another bond with C3. C3 can take both electrons from the double bond between C3-C4 and use them to form a double bond between C2-C3.
This molecule can take on these two structures because the electrons never violate an atoms octet. In this way, the molecule can “accommodate” a charge from a nearby molecule by just moving electrons:
There are a couple of key features you should be on the look out for if you are solving resonance problems.
- Lone pair of electrons on a carbon atom
- A carbocation
- A double bond between 2 atoms of different electronegativities
- Double bonds inside of a ring
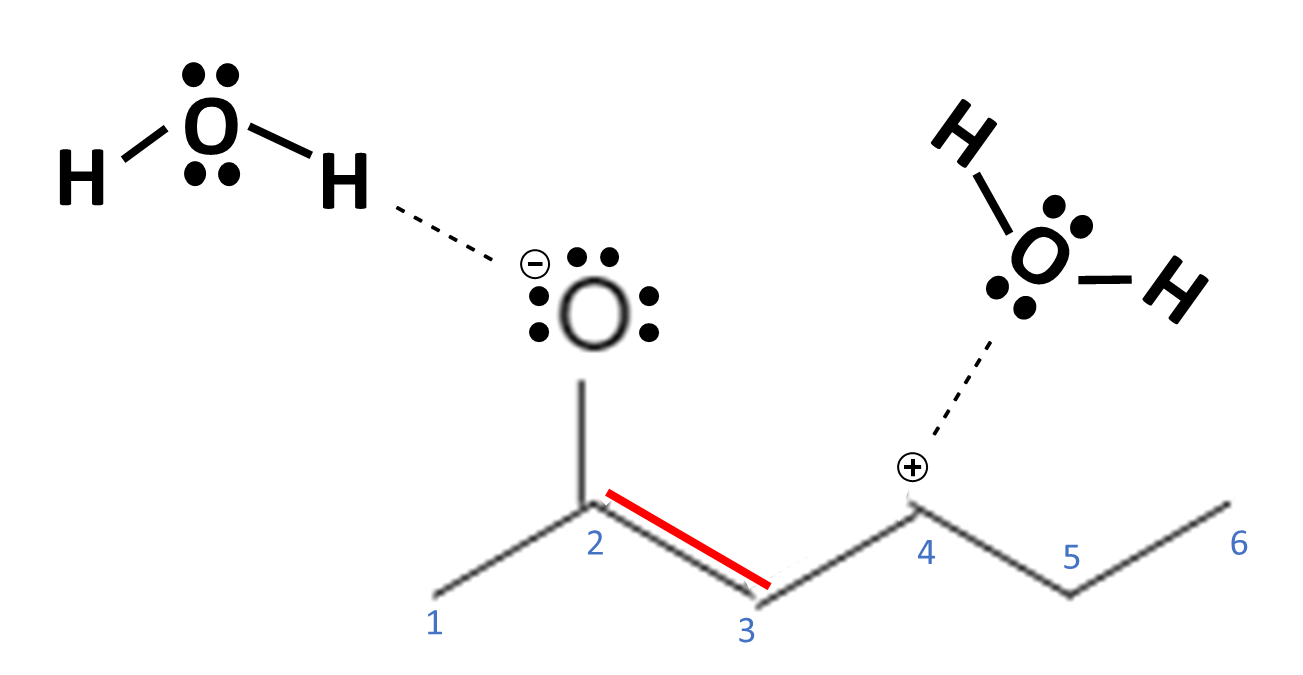
When drawing a molecule, it is best represented in the most neutral charged state as possible, representing the most stable state of the molecule. When there is a molecule that has a charge, you should draw it in the configuration where the most electronegative atom has the negative charge and the less electronegative atom has the positive charge.
In this example, the image on the left is correct and the more stable form of the molecule because O is more electronegative than C. Therefore, it can better accommodate the negative charge on the atom thereby stabilizing the whole molecule.
Resonance plays an important part in a molecule’s reactivity and stability. It is important to understand how electrons move around within a molecule because as you start to get introduced to reactions and reaction mechanisms, the order in which the electrons move becomes critical. Understanding the octet rules, formal charges, and valence electrons of the common atoms (H, C, N, O) will help you understand how bonds are being made and broken between two or more molecules.