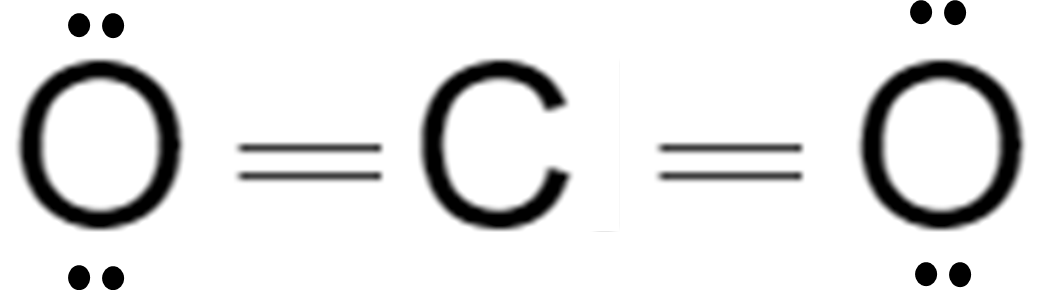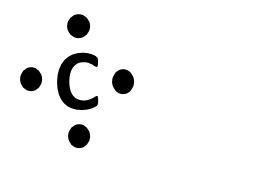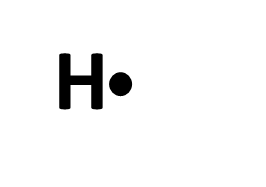Intro to Organic Chemistry
Hello students and welcome to Organic Chemistry 1! First, let’s begin with some of the basics you will need to succeed in this course. When drawing a molecule, we use lines to indicate bonds and dots to indicate electrons. This is also known as the Lewis Structure:
Carbon Dioxide
We also abbreviate atoms using their symbol from the periodic table:
Carbon (C)
Nitrogen (N)
Oxygen (O)
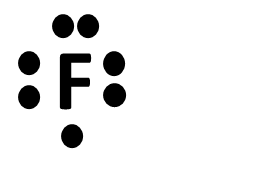
Fluorine (F), Chlorine (Cl), Bromine (Br), and Iodine (I)
Hydrogen (H)
Valence Electrons and Bonds
Molecules bond with one another by exchanging electrons, thereby forming a covalent bond. Each atom has a specific number of valence electrons, or a number of electrons that they “start with”:
C has 4 valence electrons, which allows it to make 4 bonds while maintaining a neutral charge. N has 5 valence electrons, which allows it to make 3 bonds and have 1 lone electron pair while maintaining a neutral charge.
O has 6 valence electrons, which allows it to make 2 bonds and have 2 lone electron pairs while maintaining a neutral charge.
F, Cl, Br, I have 7 valence electrons, which allows them to make 1 bond and have 3 lone electron pairs while maintaining a neutral charge.
H has 1 valence electron, but because it only has one s orbital, it only has a maximum of 2 electrons it can hold which allows it to make 1 bond.
When an atom is by itself, it only has its valence electrons:
But when an atom bonds with another atom, they each donate one electron per bond and each atom wants to complete its electron octet (or have access to 8 electrons). Hydrogen can only have 2 valence electrons; the exception to the octet rule:
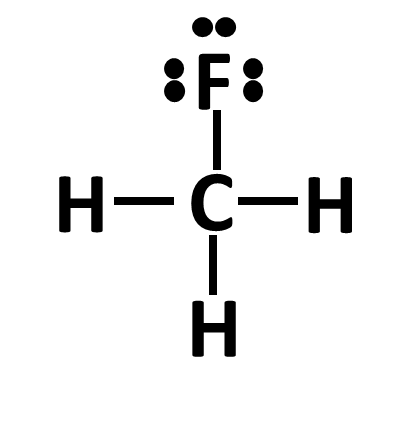
In the example above, C has 4 bonds and since each bond contains 2 electrons, C has a full octet since it has access to 8 electrons. Within each bond, 1 electron belongs to the donor and 1 belongs to the acceptor. In this case, C still has 4 electrons that belong to itself and 1 electron that is donated from each H and 1 from F. F has a full octet since it has 3 electron pairs (with 2 electrons each) and 1 bond that contributes 2 electrons; one that belongs to F and one that is shared with C.
Formal Charge
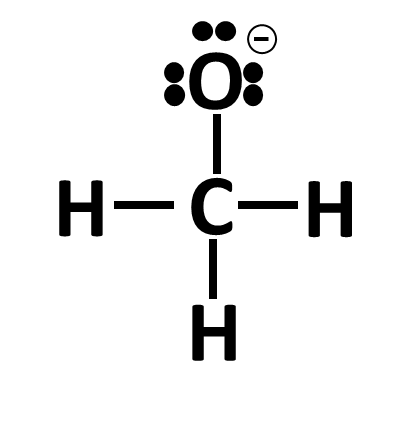
Let’s take a look at 2 examples where the octet is complete, but the atom does not have the right amount of electrons in its valence shell:
In this example, the C and O are single bonded but O has only made 1 bond. When counting how many total electrons O has, we count 8: 3 lone pair electron sets (6 electrons) and 1 bond with C (2 shared electrons). However, when we count how many electrons are in O valence shell, we get 7: 3 lone pair electron sets (6 electrons) and 1 electron from the bond with C. O needs 6 electrons in its valence shell to remain neutral and since there is a 7th electron, we draw a negative charge next to it (remember: electrons are negatively charged so having an extra electron adds a negative charge, being short an electron gives a positive charge).
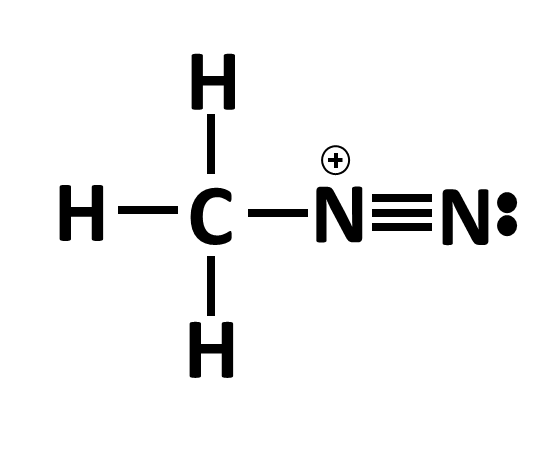
Let’s look at another example:
In this example, the N on the far right has 3 bonds with the N in the middle (6 electrons) and also has 1 lone pair electron set (2 electrons) which completes its octet. When counting the valence electrons to the far right N, we count 5: 2 electrons from the lone pair and 3 electrons from the bonds (1 electron from each bond belongs to N). This gives the far right N a neutral charge. When looking at the middle N, we see that there are no lone pairs but that N has 4 bonds (3 to the far right N and 1 to the C). This means that N has a complete octet (has access to 8 electrons) but when counting the valence electrons, there are only 4 electrons that the middle N can call its own. N needs to have 5 electrons in its valence shell to maintain a neutral charge. Since this N only has 4, it has a positive charge since it is short one electron (and electrons have negative charges, so being short one electron yields a positive charge).