Acid–Base Chemistry in Organic Molecules
Acid–Base Chemistry in Organic Molecules
Acid–base reactions dominate early organic chemistry, governing everything from proton transfers in synthesis to mechanistic selectivity.
1.1 Brønsted–Lowry definitions
- Acid (HA): proton donor
- Base (B): proton acceptor
- Conjugate Acid (A-): proton acceptor
- Conjugate Base (HB+): proton acceptor
These terms always describe a pair of substances that differ by exactly one proton. In any acid–base reaction, the acid on one side has a related conjugate base on the same side, and the base has a related conjugate acid.
Example: acetic acid and sodium acetate
Consider acetic acid (CH₃COOH), the main acid in vinegar:
CH₃COOH ⇌ CH₃COO⁻ + H⁺
(acid) (conjugate base)
-
Acid: CH₃COOH donates a proton to become acetate.
-
Conjugate base: CH₃COO⁻ is what remains after the proton is donated.
- If acetate encounters a strong acid, it can accept a proton and revert to acetic acid, acting as a base in that context.
In everyday life, this acid/conjugate base pair is important for food safety and preparation. For example:
-
Acetic acid in vinegar is acidic enough to inhibit microbial growth, making it a preservative in pickling.
-
Sodium acetate (the sodium salt of acetate) acts as a mild base and a buffering agent, helping stabilize pH in certain recipes and packaged foods.
1.2 Acid dissociation equilibrium
When an acid dissolves in water, it can donate a proton (H⁺) to water or to another molecule.
This process is called acid dissociation.
What is Kₐ?
The acid dissociation constant, written Kₐ, is a numerical measure of how much an acid dissociates into ions in water at equilibrium.
It’s based on the concentrations of the products and reactants once the system has settled (equilibrium).
For a generic acid HA:
- HA = the undissociated acid molecule
- H⁺ = the proton (in water, this exists as H₃O⁺, the hydronium ion)
- A⁻ = the conjugate base of the acid
The equilibrium expression for Kₐ is:
And the relationship between pKa and Ka is described in the equation below:
Low pKₐ → strong acid · High pKₐ → weak acid
- Low pKₐ (≈ 0 or less) → very strong acid
- Moderate pKₐ (~ −1 to 5) → e.g., carboxylic acids
- High pKₐ (> 15) → weak acids like alcohols and amines
Key point:
The smaller the pKₐ, the stronger the acid, because a smaller pKₐ means a larger Kₐ and greater proton donation.
1.3 Base dissociation equilibrium
Just as acids have an equilibrium constant Kₐ for donating protons, bases have an equilibrium constant Kb for accepting protons.
- Base dissociation constant (Kb): measures the extent to which a base reacts with water to produce its conjugate acid and hydroxide ion.
- pKb: the negative logarithm of Kb.
For a generic base B:
B = the base molecule (can accept a proton)
H₂O = acts as the proton donor in this equilibrium
BH⁺ = the conjugate acid of the base
OH⁻ = hydroxide ion produced when the base accepts a proton
The equilibrium expression for Kb is:
And the relationship between pKb and Kb is described in the equation below:
Low pKb → strong base · High pKb → weak base
A low pKb value means the base is strong (reacts readily with water), while a high pKb means the base is weak.
2. Conjugate acid–base pairs & arrow-pushing mechanics
In arrow‑pushing notation, a curved arrow always shows the movement of a pair of electrons during a reaction step.
Arrows start from electrons — never from the hydrogen atom itself.
For example, in the reaction between acetic acid (CH₃COOH) and water:
| HA | + | B | ⇌ | A⁻ | + | BH⁺ |
| (acid) | (base) | (base) | (acid) |
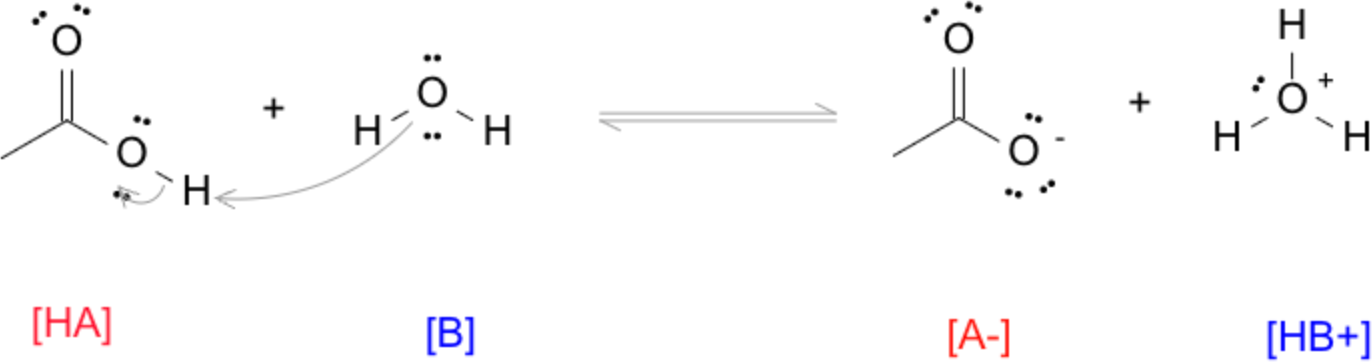
- The arrow begins at the lone pair of electrons on the oxygen atom in water (the base).
- It points toward the hydrogen atom on the hydroxyl group (–OH) of acetic acid.
- A second arrow starts at the O–H bond in acetic acid and points back to the oxygen atom of the acid, showing that this bond pair becomes a lone pair when the proton leaves.
This notation captures the electron‑flow pathway:
- The base’s electrons form a new bond to the proton.
- The acid’s O–H bond breaks, with the electrons staying on oxygen to form the conjugate base.
By always starting arrows from electron sources (lone pairs or π bonds), you demonstrating your knowledge that electron density drives chemical change.
3. Authoritative pKₐ table
Use this as a quick reference for predicting acid–base equilibria. Lower pKₐ means stronger acid.
| Functional group | Typical pKₐ | Conjugate base |
|---|---|---|
| mineral acids (HCl, H₂SO₄) | < 0 | Cl⁻, HSO₄⁻ |
| protonated carbonyls (R–C=O–H⁺) | −6 to −4 | neutral carbonyl |
| carboxylic acids (R–COOH) | ~ 4.5–5.0 | R–COO⁻ |
| protonated amines (R₃NH⁺) | ~ 10.5 | R₃N |
| phenols (Ar–OH) | ~ 10 | Ar–O⁻ |
| alcohols (R–OH) | ~ 16 | R–O⁻ |
| water (H₂O) | 15.7 | OH⁻ |
| α‑hydrogens of carbonyls | 18–20 | enolate |
| terminal alkynes (RC≡CH) | ~ 25 | acetylide |
| alkenes (C=C–H) | ~ 44 | vinyl carbanion |
| alkanes (C–H) | ≥ 50 | alkyl carbanion |
4. Factors governing organic acid strength
Organic acid strength correlates directly with the stability of the conjugate base (A⁻).
| Factor | Effect on A⁻ stability | Resulting pKₐ trend |
|---|---|---|
| electronegativity | More electronegative atom stabilizes negative charge | ↓ pKₐ |
| resonance delocalisation | Charge spread over multiple atoms stabilizes base | ↓ pKₐ |
| inductive effects | Electron‑withdrawing groups pull e⁻ density | ↓ pKₐ |
| hybridisation | Greater s‑character localises electrons near nucleus |
sp < sp² < sp³ in acidity strength |
5. Worked examples
5.1 predicting equilibrium direction
Reaction: CH₃NH₂ + CH₃COOH ⇌ CH₃NH₃⁺ + CH₃COO⁻
pKₐ(CH₃COOH) = 4.76
pKₐ(CH₃NH₃⁺) = 10.6
Lower pKₐ acid is stronger; here acetic acid is stronger. Equilibrium favors the side with the weaker acid → products (right side)
5.2 base selection for alkyne deprotonation
Substrate: HC≡CH (pKₐ ≈ 25)
Required base: conjugate acid pKₐ > 25.
NaNH₂ (pKₐ NH₃ ≈ 38) succeeds; NaOH (pKₐ H₂O ≈ 15.7) fails.
5.3 ranking acidity
Order these by acidity: cyclohexanol, 2‑chlorophenol, phenol.
pKₐ: 2‑chlorophenol ≈ 8.5 < phenol ≈ 10 < cyclohexanol ≈ 16.
Most acidic: 2‑chlorophenol (EWG + resonance).
6. practice problems
- Predict equilibrium direction for acetone (pKₐ ≈ 19.2) + tert‑butanol (pKₐ ≈ 18).
- Identify the most acidic proton in ethyl acetoacetate (CH₃COCH₂COOEt) and explain.
- Arrange by increasing acidity: methane, ethene, ethyne.
solutions
1. tert‑butanol acid pKₐ = 18 < 19.2 → tert‑butanol stronger acid → equilibrium to the left.
2. α‑CH₂ between two carbonyls → resonance‑stabilized enolate, pKₐ ≈ 11. Labeled as Carbon 1
3. CH₄ (~50) < C₂H₄ (~44) < C₂H₂ (~25).