Isomerism and Stereochemistry
Isomerism and Stereochemistry
Organic compounds are compounds that contain carbon atoms bonded to other atoms such as hydrogen, oxygen, nitrogen, and sulfur. One of the fundamental properties of organic compounds is their ability to form isomers. Isomers are molecules with the same molecular formula but different structures and properties. Understanding isomerism and stereochemistry is crucial to organic chemistry since different isomers can have different biological and chemical properties.
Structural Isomers
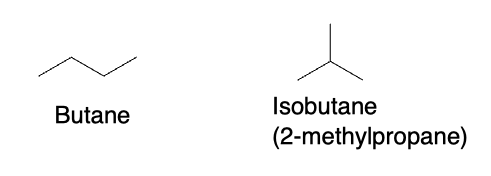
Structural isomers are isomers that have the same molecular formula but differ in their connectivity. In other words, structural isomers have different arrangements of atoms in their molecular structure. The simplest example of structural isomers is butane and isobutane. Butane has a linear structure, while isobutane has a branched structure:
Constitutional Isomers
Constitutional isomers are another name for Structural Isomers. Another example of constitutional/structural isomers is pentane and 2-methylbutane:

Stereoisomers
Stereoisomers are isomers that have the same molecular formula and connectivity, but differ in the arrangement of atoms or groups in three-dimensional space. Stereoisomers can be further divided into two categories: enantiomers and diastereomers.
Enantiomers
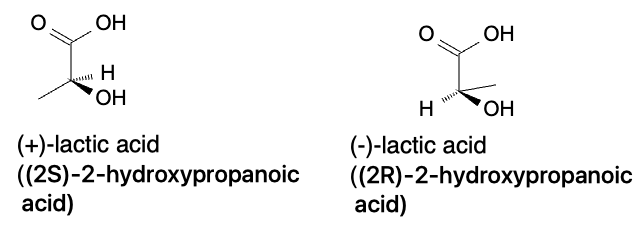
Enantiomers are stereoisomers that are non-superimposable mirror images of each other. They have the same physical and chemical properties except for their interaction with plane-polarized light. One enantiomer will rotate plane-polarized light clockwise (dextrorotatory or +) while the other rotates the plane counterclockwise (levorotatory or -). Enantiomers are commonly found in biological systems and can have different physiological effects. One famous example of enantiomers is the drug thalidomide, which was prescribed in the 1950s and 1960s to treat morning sickness in pregnant women. One enantiomer of thalidomide was found to be an effective sedative, while the other enantiomer caused severe birth defects.
Diastereomers
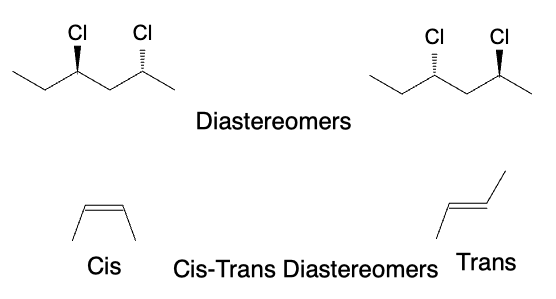
Diastereomers are stereoisomers that are not mirror images of each other. They have different physical and chemical properties and can have different biological activities. Cis-trans isomers and meso compounds are examples of diastereomers. Cis-trans isomers are diastereomers that have different arrangements of substituent groups around a double bond. Meso compounds are compounds that have chiral centers but are achiral due to internal symmetry.
Summary
Isomerism and stereochemistry are fundamental concepts in organic chemistry. Isomers can have different properties and reactivity, so understanding their structure and properties is essential for predicting their behavior in reactions. Stereochemistry is particularly important in biochemistry, where the stereochemistry of molecules can influence their interaction with enzymes, receptors, and other biomolecules. Mastery of isomerism and stereochemistry is critical in designing and developing new drugs, materials, and chemical processes.
Test Your Knowledge:
-
What are the different types of isomers? Give an example of each.
-
What is the difference between enantiomers and diastereomers? Give an example of each.