Structure and Bonding
Structure and Bonding
Understanding the structure and bonding of molecules is fundamental to organic chemistry. Chemical bonds hold atoms together in molecules, and the way atoms bond determines the structure and properties of a compound. This lesson reviews atomic structure and the two primary bond types—ionic and covalent—plus the octet rule, electronegativity, and bond polarity.
Quick Guide
- Atoms, Electrons, and the Octet Rule
- Ionic vs. Covalent Bonds
- Electronegativity and Polar Bonds
- Summary
Atoms, Electrons, and the Octet Rule
Atoms consist of a nucleus (protons and neutrons) surrounded by electrons in discrete atomic orbitals. The most important electrons for bonding are the valence electrons, which reside in the atom's outermost shell. Atoms tend to undergo reactions that result in a full valence shell (often eight electrons for main-group elements), a tendency known as the octet rule. Achieving a stable noble gas electron configuration drives atoms to form chemical bonds.
- Valence Electrons: The electrons in the highest energy level of an atom. For example, carbon (atomic number 6) has four valence electrons (2s²2p²) and needs four more to complete an octet.
- Octet Rule: A rule of thumb stating that main-group elements often form bonds until they are surrounded by eight valence electrons. Hydrogen is an exception, aiming for two electrons (duet rule), while elements like boron or phosphorus can have exceptions (incomplete or expanded octets).
When atoms bond, they either transfer or share electrons to satisfy the octet rule. This leads to the two primary bond types: ionic bonds and covalent bonds.

Ionic vs. Covalent Bonds
Ionic bonds form through the transfer of electrons from one atom to another, creating charged ions that attract each other. Typically, a metal atom loses one or more electrons to become a positively charged cation, while a nonmetal gains those electrons to become a negatively charged anion. The electrostatic attraction between oppositely charged ions results in an ionic bond. A classic example is sodium chloride (NaCl), where sodium (Na) donates an electron to chlorine (Cl), producing Na⁺ and Cl⁻ ions held together in a lattice by ionic attraction.
Covalent bonds, in contrast, involve the sharing of electron pairs between atoms. Nonmetal atoms (such as carbon, hydrogen, oxygen, and nitrogen in organic molecules) commonly form covalent bonds by sharing electrons so that each atom attains a full valence shell. For instance, two hydrogen atoms can share their electrons to form an H–H bond, giving each hydrogen access to two electrons total. Carbon, with four valence electrons, forms four covalent bonds (as in methane, CH₄) to achieve a stable octet.
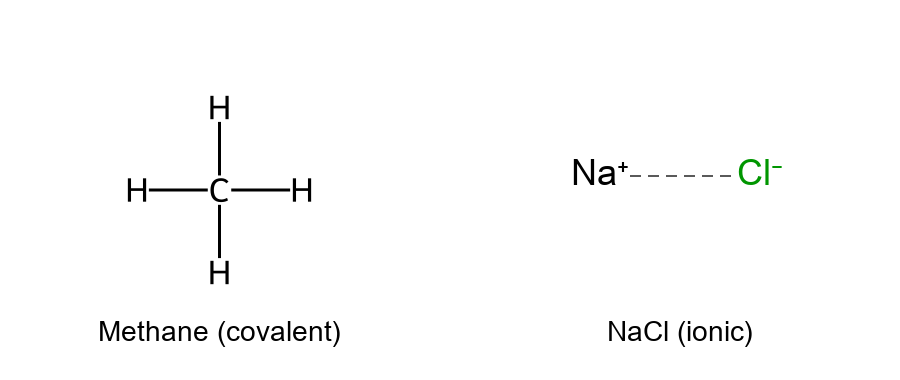
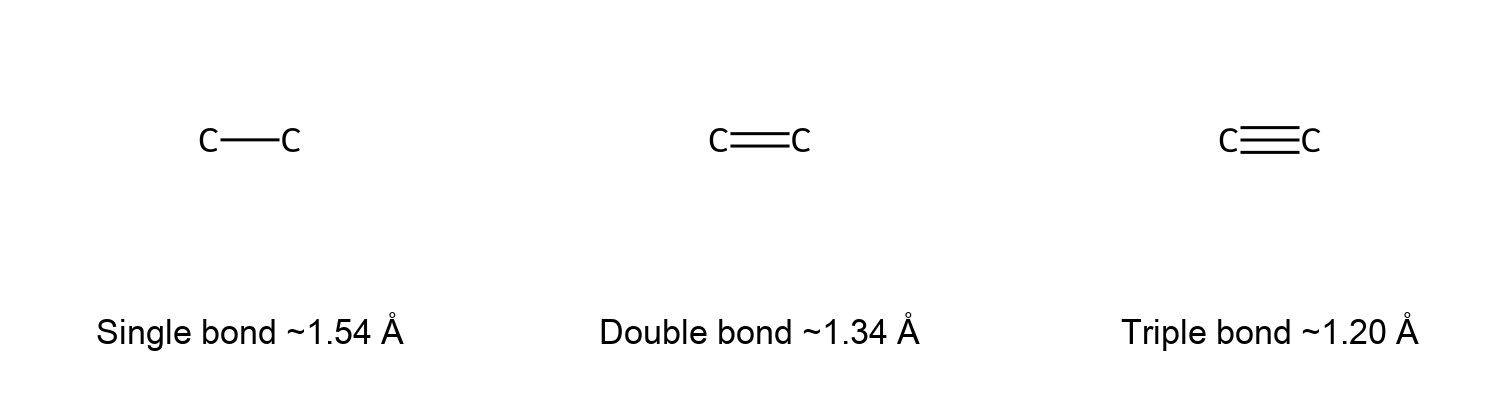
- Single, Double, Triple Bonds: Covalent bonding can involve one shared pair of electrons (a single bond), two shared pairs (a double bond), or three shared pairs (a triple bond). A single bond (e.g., C–C in ethane) is a pair of shared electrons. A double bond (e.g., C=C in ethene) consists of two pairs shared, and a triple bond (e.g., C≡C in ethyne) has three shared pairs. Multiple bonds are shorter and stronger than single bonds.
- Bond Strength and Length: Generally, triple bonds are stronger and shorter than double bonds, which in turn are stronger and shorter than single bonds. Covalent bond strength is also influenced by the specific atoms involved.
In organic chemistry, covalent bonding is predominant since organic compounds are primarily made of nonmetals. Carbon's ability to form four covalent bonds (tetravalency) is what allows the vast diversity of organic molecules.
Electronegativity and Polar Covalent Bonds
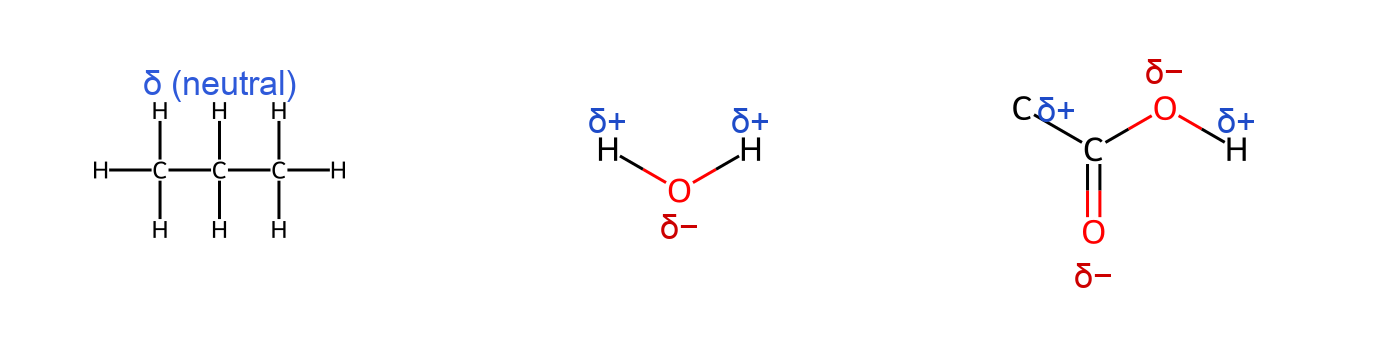
While covalent bonds involve sharing electrons, the sharing is not always equal. Electronegativity is a measure of how strongly an atom attracts electrons in a bond. When two atoms in a covalent bond have different electronegativities, the electron pair is drawn closer to the more electronegative atom, creating a polar covalent bond. One end of the bond becomes slightly negative (δ⁻) and the other end slightly positive (δ⁺), forming a dipole. For example, in a water molecule (H₂O), oxygen is more electronegative than hydrogen. The O–H bonds are polar, with electrons pulled toward oxygen, giving the oxygen a partial negative charge and each hydrogen a partial positive charge. In contrast, bonds between identical atoms (like H–H or C–C) share electrons evenly and are nonpolar.
-
Nonpolar Covalent Bond: A bond between atoms of equal or very similar electronegativity, resulting in an even distribution of electron density (e.g., C–H bonds are nearly nonpolar due to a small electronegativity difference).
-
Polar Covalent Bond: A bond between atoms with a noticeable electronegativity difference, causing unequal sharing of electrons (e.g., C–O or H–Cl bonds are polar). The greater the electronegativity difference, the more polar the bond.
Bond polarity has important consequences in molecular interactions and reactivity. Polar bonds can lead to dipole moments in molecules, affecting properties like boiling point and solubility. For instance, water’s polar bonds and bent structure result in a strong molecular dipole, making it a polar molecule overall.
Summary
Chemical bonding is driven by atoms’ tendency to achieve stable electron configurations. Ionic bonds arise from electron transfer between metals and nonmetals, forming oppositely charged ions, whereas covalent bonds involve sharing electrons between nonmetals. Carbon’s four valence electrons make it uniquely suited to form four covalent bonds, underlying the complexity of organic structures. Bonding can be further understood in terms of polarity: differences in electronegativity lead to polar covalent bonds with unequal electron sharing, while identical atoms form nonpolar bonds.
- Chemical bonding follows the drive to complete valence shells (octet/duet).
- Ionic bonds form from electron transfer; covalent bonds form from electron sharing.
- Electronegativity differences create bond polarity; similar atoms yield nonpolar bonds.