How do I know if a molecule is Aromatic, Antiaromatic, or Not Aromatic?
Aromatic or Anti-Aromatic? Understanding the Key Differences
To find out if a molecule is aromatic, antiaromatic, or not aromatic, use our Aromatic Ring Detector tool!
Aromaticity is one of the most intriguing topics in organic chemistry, often leaving students wondering: Is this molecule aromatic or anti-aromatic? This question lies at the heart of understanding the stability and reactivity of cyclic compounds. Historically tied to fragrant substances, the term “aromatic” now describes a unique electronic property that provides remarkable stability to some molecules. In this article, we will break down the criteria for aromaticity and anti-aromaticity, demystifying the rules that govern their classification.
Criteria for Aromaticity
For a molecule to be aromatic, it must satisfy the following four criteria, known as Hückel’s rules:
-
Cyclic Structure: The molecule must form a closed ring of atoms.

-
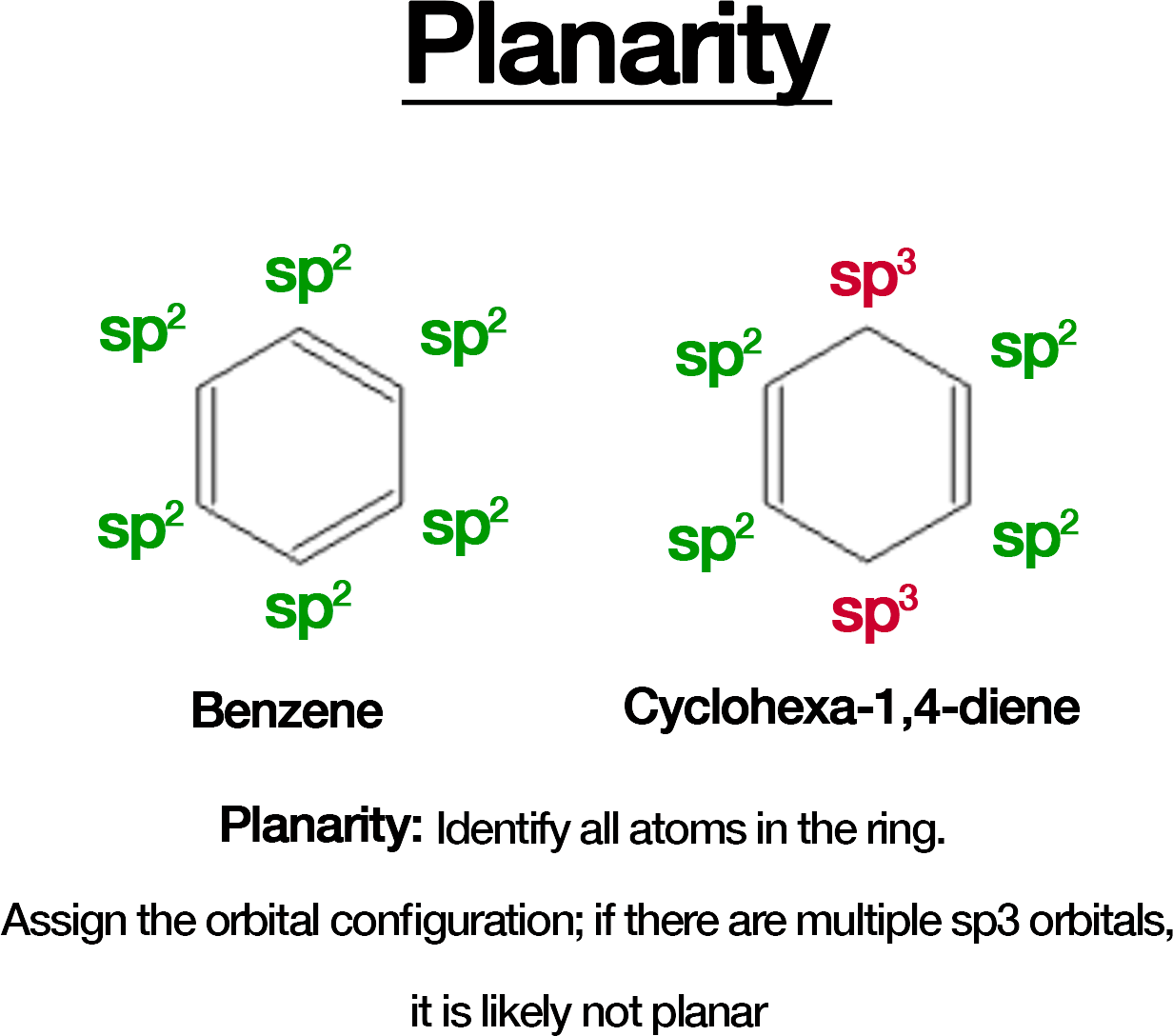
Planarity: The ring must be planar (all atoms in the ring lie in the same plane).
Evaluate the Atom Types: Atoms with sp2 hybridization in a cyclic structure are typically planar. Watch for sp3-hybridized atoms, which can disrupt planarity.
Quick and dirty rule: sp3 atoms have no double bonds, sp2 atoms have at least 1 double bond.
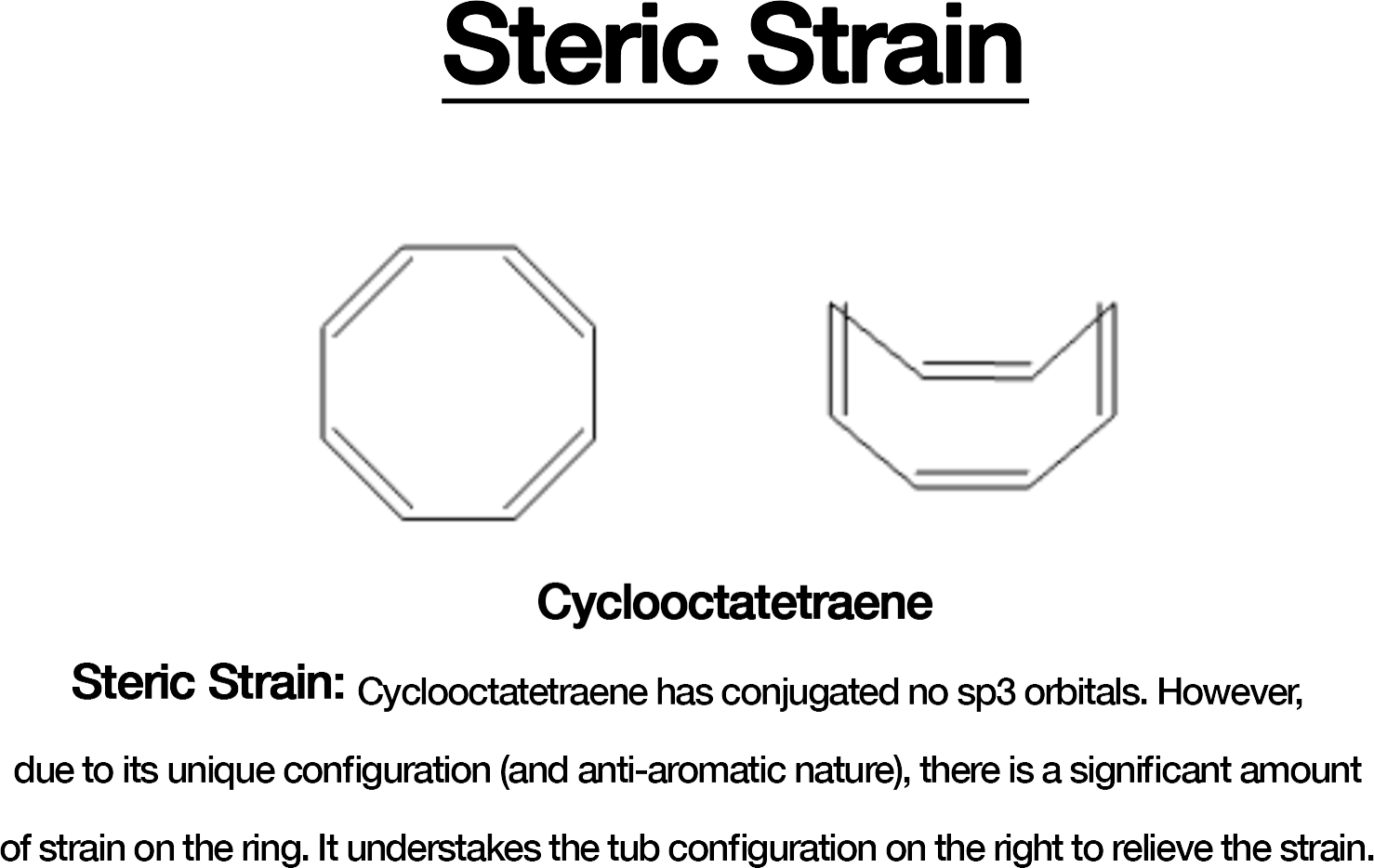
Look for Steric Hindrance: Bulky substituents or structural strain can cause the ring to twist, making it non-planar. For example, in cyclooctatetraene, steric strain forces a tub-shaped geometry.
Use Provided Data: If bond angles, resonance structures, or molecular models are provided, check if all atoms can lie in the same plane without significant distortion.
-
Conjugation: The molecule must have a continuous overlap of p-orbitals (it must be fully conjugated).

-
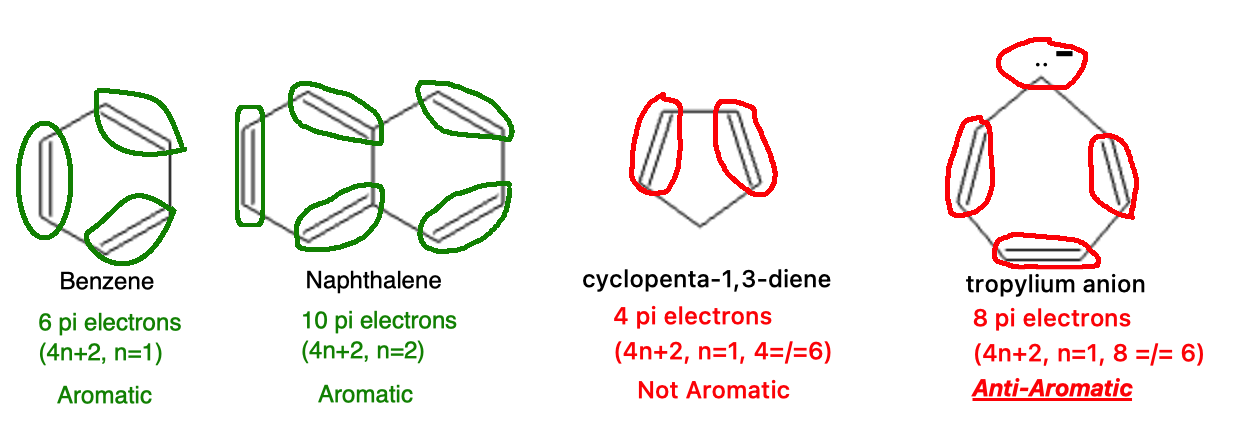
Hückel’s Rule: The molecule must have 4n+2 electrons, where “n” is a non-negative integer (0, 1, 2, 3…) which results in a total number of electrons following the pattern: 2, 6, 10, 14…
Anti-Aromatic Compounds
Anti-aromatic compounds share the first three criteria of aromatic compounds: they are cyclic, planar, and fully conjugated. However, they fail Hückel’s rule because they have 4n electrons (e.g., 4, 8, 12 electrons). This electron configuration leads to instability due to significant electronic repulsion and the inability to achieve a delocalized, low-energy state.
Characteristics of Anti-Aromatic Compounds:
- Instability: Anti-aromatic molecules are highly unstable compared to their aromatic or non-aromatic counterparts. Planar Conjugation: Despite their instability, they maintain planarity and conjugation.
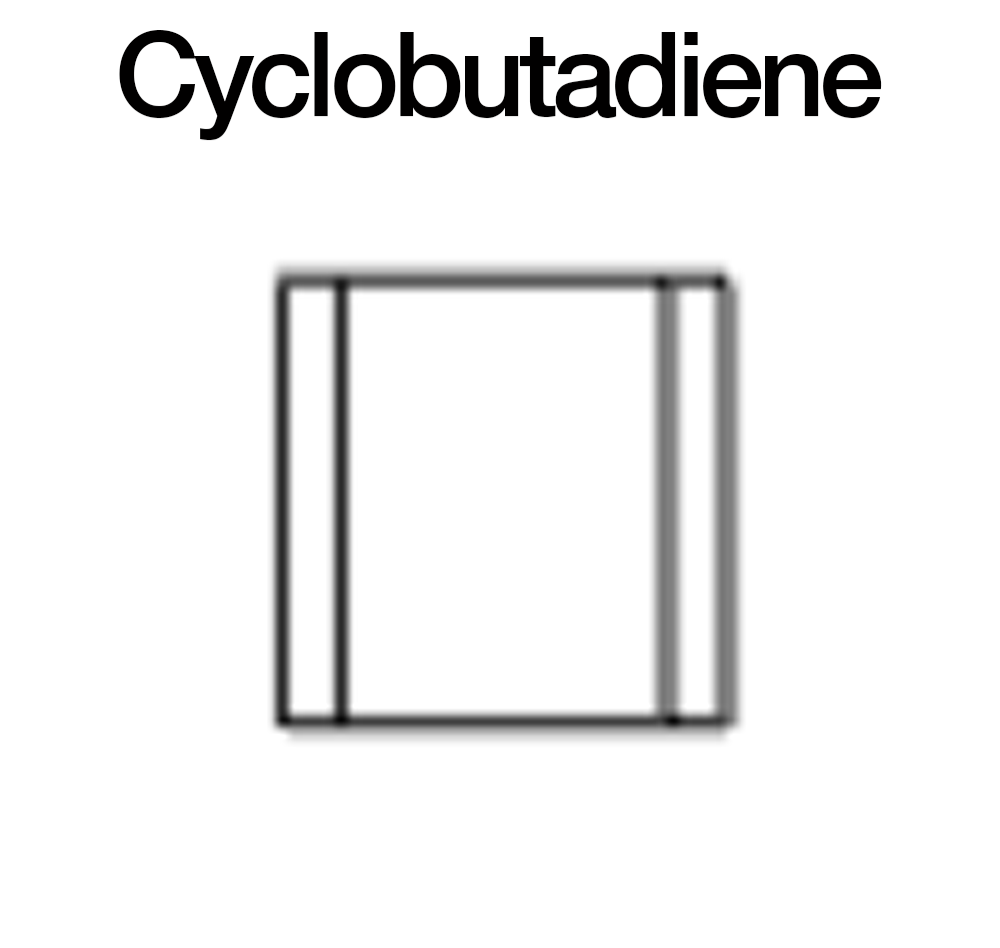
An example of an anti-aromatic compound is cyclobutadiene, which has 4 pi electrons. This molecule is so unstable that it typically dimerizes or adopts a distorted structure to relieve anti-aromaticity.
Non-Aromatic Compounds
Non-aromatic compounds fail one or more of the criteria for aromaticity. These molecules may be non-cyclic, non-planar, or lack conjugation. They do not experience the extraordinary stability of aromatic compounds or the instability of anti-aromatic ones. Example of Non-Aromaticity:
Cyclohexane, a non-cyclic alkane, lacks -electrons and conjugation, making it non-aromatic.
Differentiating Aromatic and Anti-Aromatic Molecules
To determine whether a compound is aromatic, anti-aromatic, or non-aromatic, follow these steps:
- Check for a Ring Structure:
Is the molecule cyclic? If not, it is non-aromatic.
- Assess Planarity:
Are all atoms in the ring planar? If not, the molecule is non-aromatic.
- Evaluate Conjugation:
Does the molecule have continuous overlap of p-orbitals (i.e., fully conjugated)? If not, it is non-aromatic.
- Apply Hückel’s Rule:
Count the electrons in the molecule: If the number fits 4n+2, it is aromatic. If the number fits 4n, it is anti-aromatic.
What Makes a Molecule Anti-Aromatic?
The distinguishing feature of anti-aromatic molecules is their electron configuration (“ -electrons”). This leads to: Electron-Electron Repulsion: The electrons in the -system cannot delocalize efficiently, leading to high energy. Lack of Stabilization: Unlike aromatic systems, anti-aromatic compounds cannot achieve a delocalized, lower-energy state.
Common Exam Traps
When tackling exam questions related to aromaticity, students often encounter tricky scenarios that test their understanding of key concepts. Here are the most common traps and strategies to avoid them:
- Miscounting Electrons:
One of the most frequent errors is miscalculating the number of electrons in a system. Remember: Lone pairs in a conjugated system may count as -electrons if they participate in resonance. Double bonds contribute 2 electrons each. Don’t include electrons or unshared lone pairs that are not part of the conjugated system.
- Forgetting Planarity:
Planarity is crucial for aromaticity and antiaromaticity. Molecules like cyclooctatetraene may appear to satisfy the “4n” or “4n+2” rule but, they adopt a non-planar, tub-shaped geometry to avoid antiaromaticity. Always consider the molecule’s geometry.
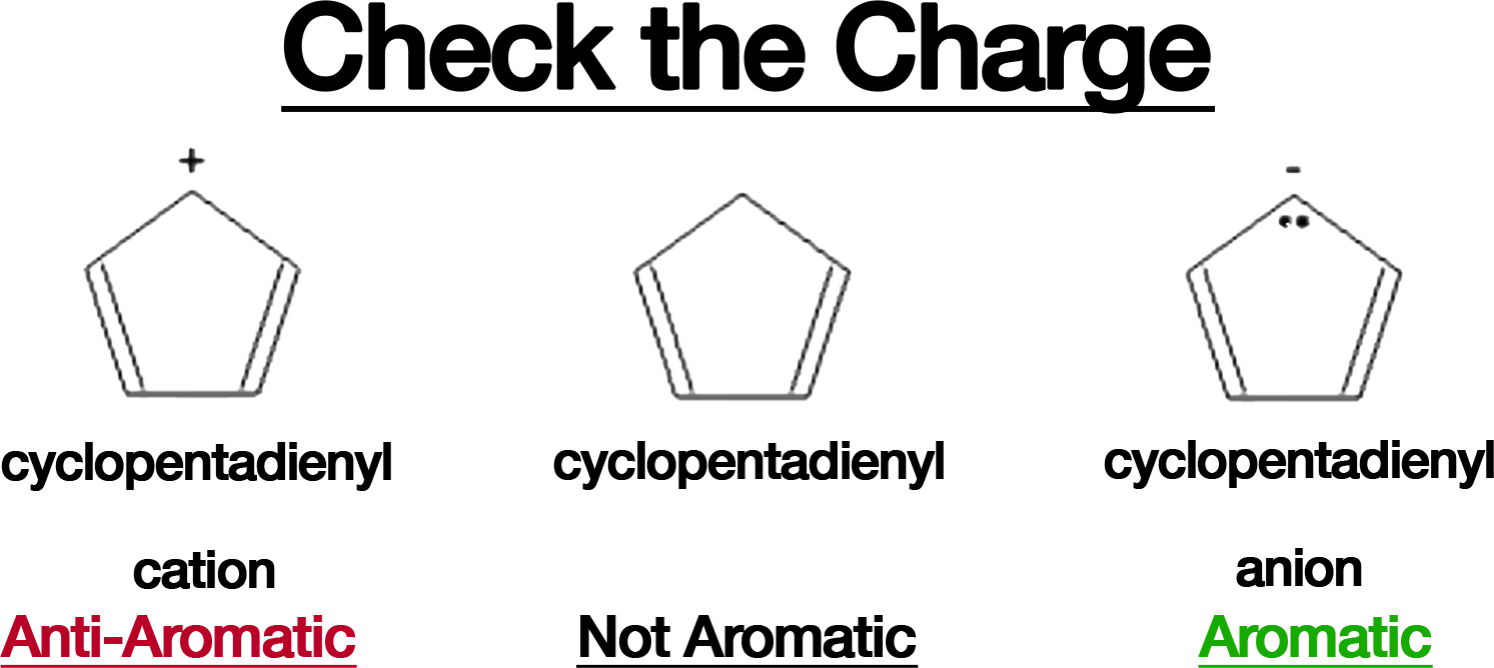
- Overlooking the Charge:
Ions can significantly alter aromaticity. For example: The cyclopentadienyl anion (-) is aromatic with 6 -electrons. The cyclopentadienyl cation (+) has only 4 -electrons and is anti-aromatic. Pay attention to the formal charges when analyzing a compound’s electronic structure.
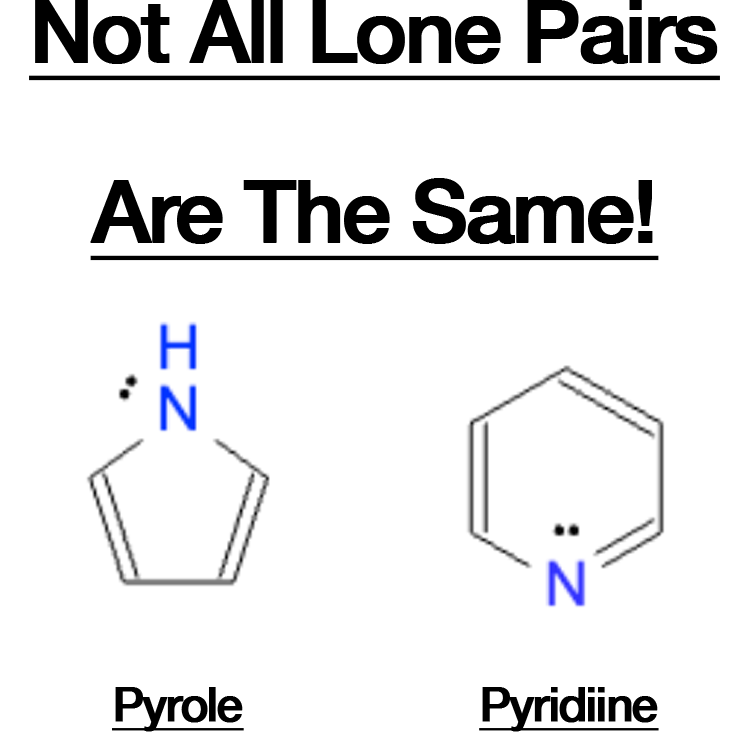
- Misinterpreting Lone Pair Contributions: Heterocyclic compounds often confuse students. For instance: In pyrrole, the lone pair on nitrogen is part of the aromatic system, giving it 6 electrons, making it aromatic. In pyridine, the lone pair of nitrogen does not participate in the aromatic system, leaving it aromatic with 6 electrons from the double bonds alone. Carefully evaluate lone pairs to determine if they contribute to the conjugated system.
- Misclassifying Non-Aromatic Molecules:
Some cyclic compounds fail to meet criteria for both aromaticity and anti-aromaticity. For example: Cycloheptatriene is non-aromatic due to its localized double bonds and inability to maintain planarity. Distinguish these cases carefully by checking all criteria.
- Failing to Apply Hückel’s Rule Correctly:
Hückel’s rule (“4n+2”) is a cornerstone of aromaticity, but students often misapply it. Ensure: is a non-negative integer (e.g., 0, 1, 2, etc.). Only count electrons from conjugated -systems within a planar ring. Avoid confusing the “4n” rule for anti-aromaticity with the “4n+2” rule.
Conclusion
Aromaticity is a fundamental concept in organic chemistry, serving as the key to understanding why certain cyclic molecules are remarkably stable while others are highly reactive. By mastering the ability to classify compounds as aromatic, anti-aromatic, or non-aromatic, students and chemists alike can accurately predict chemical behavior and reactivity. This knowledge is indispensable for success in organic chemistry exams and has broad applications in reaction mechanisms, material science, and molecular design. Whether you’re solving homework problems or designing novel compounds, understanding aromaticity is the cornerstone of excelling in organic chemistry.