Introduction to Aromatic and anti-Aromatic Compounds
Aromatic compounds are an essential topic in organic chemistry, characterized by their unique stability and distinct electronic structure. Aromaticity refers to a property of certain cyclic compounds that possess a higher stability and specific chemical reactivity. In this lesson, we will explore the concept of aromaticity, learn how to identify aromatic compounds, and understand the significance of aromaticity in benzene and other compounds.
Definition of Aromaticity
Aromaticity is a property exhibited by cyclic compounds that possess a specific arrangement of π-electrons, resulting in enhanced stability and unique reactivity.
A compound is considered aromatic if it follows Huckel's rule:
Huckel's Rule
Huckel's rule, named after Erich Hückel, is a concept in organic chemistry that helps predict the aromaticity of cyclic conjugated systems. According to Huckel's rule, a compound is considered aromatic if it meets the following criteria:
The compound must be cyclic: It should have a closed loop of atoms, forming a ring structure:

In this example, benzene is cyclic (complete ring structure), (3Z)-hexa-1,3,5-triene is not cyclic (ring is open), and while cyclohexane has its ring structure intact, it does not follow the rules below, which will explain why it is not aromatic.
The compound must be planar: The atoms in the ring should lie in a single plane.
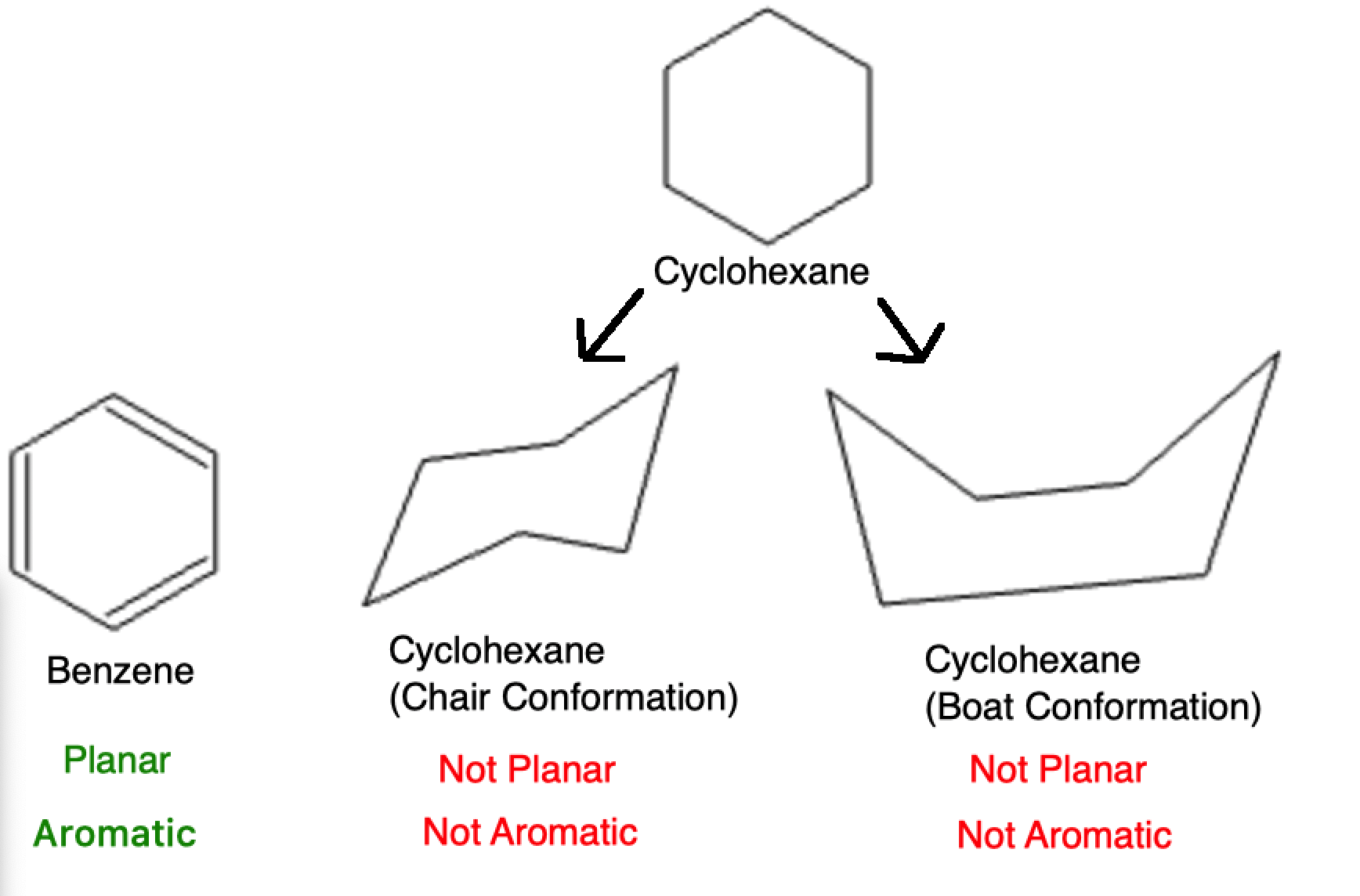
In this example, the benzene atoms all lay in a single plane due to the pi bonding. As a flashback to Organic Chemistry 1 lessons, hexane can take on multiple conformations such as the Chair and Boat conformation. These conformations result in some atoms not being on the same plane as others, which precludes it from being an aromatic molecule.
The compound must have a continuous system of conjugated pi (π) electrons: This means that there should be alternating single and multiple bonds (usually double bonds) throughout the ring.

In this example, naphthalene has a series of pi bonds that alternate throughout the ring structue. Cyclohexane only has 1 pi bond, which does not satisfy this rule.
The compound must have a total number of π electrons equal to 4n+2, where n is an integer: Aromatic compounds have a specific number of π electrons that fulfill this equation, making the system particularly stable. The "4n+2" rule is often referred to as the "Huckel's rule."
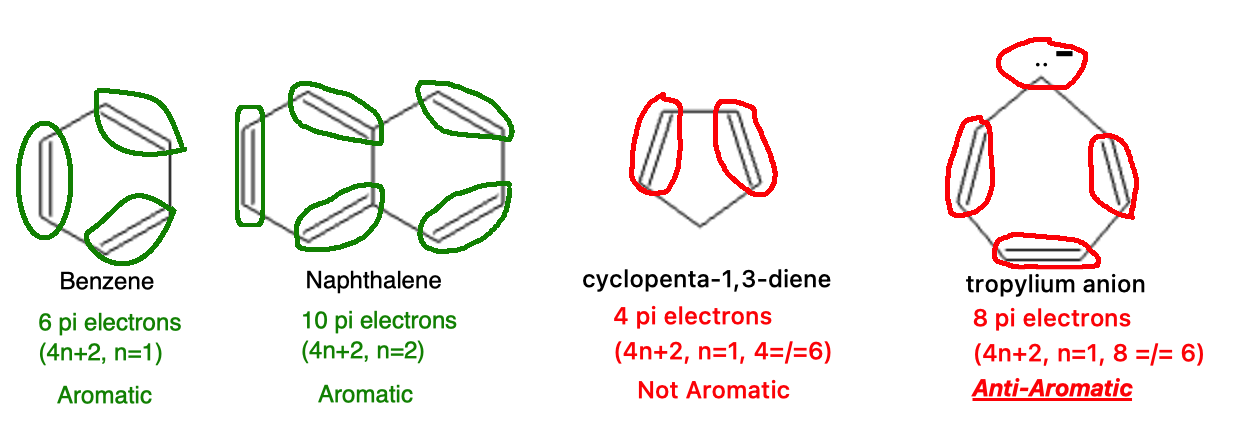
The 4n+2 rule uses any integer (whole number) as the "n" (1,2,3...). In this example, we see benzene once again. Benzene has 6 pi electrons (3 double bonds, 2 electrons per double bond) which satisfies Huckel's rule (n=1, 4n+2 = 6, benzene = 6). Next is naphthalene with 10 pi electrons (n=2, 4n+2 = 10, naphthalene = 10). Next is cyclopenta-1,3-diene which has 4 pi electrons, which will not meet the mathematical rule. Finally we have the tropylium anion; this has 3 double bonds (6 electrons) and 1 lone pair of electrons (2 electrons) resulting in 8 total electrons. This will not fit into the 4n+2 rule however, it does fit in to the 4n rule, which is part of definining anti-aromaticity.
If a cyclic conjugated system meets all of these criteria, it is considered aromatic. Aromatic compounds are known for their stability, lower reactivity, and distinct properties compared to non-aromatic or antiaromatic compounds. Huckel's rule provides a useful guideline for predicting and understanding the aromaticity of various organic compounds.
Antiaromaticity
Antiaromaticity is the opposite of aromaticity and refers to a property exhibited by certain cyclic compounds that have a continuous system of conjugated π electrons but fail to meet the criteria of Huckel's rule. Antiaromatic compounds are generally less stable and possess higher reactivity compared to non-aromatic or aromatic compounds.
In contrast to the 4n+2 rule for aromaticity, antiaromatic compounds have a total number of π electrons that satisfies the 4n rule. This means they possess an even number of π electrons within their conjugated system. This electron count leads to a destabilizing effect due to the increased electron-electron repulsion within the cyclic structure.
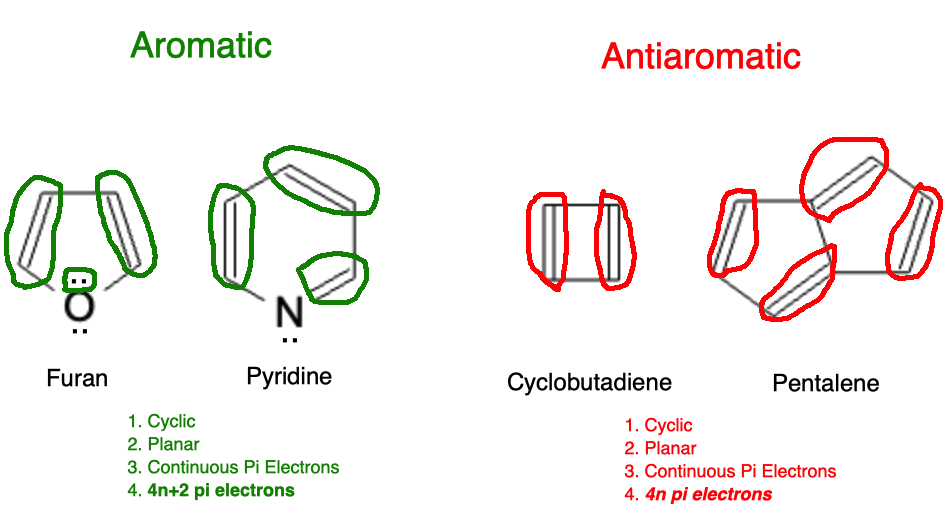
In this example, we see furan and pyridine satisfy all requirements of an aromatic molecule. Of note, the oxygen atom on furan has 2 lone pairs of electrons however, only 1 of those two lone pairs is in a p orbital; the other is sp2 hybridized. Each atom in a ring can only contribute 1 p orbital to the aromatic system.
For the antiaromatic molecules, we see that they are cyclic, planar, have continuous pi electrons, but only have 4n pi electrons (cyclobutadiene has 4 pi electrons and pentalene has 8 pi electrons). This makes them antiaromatic and they will be much less stable than their aromatic counterparts
Summary
Aromaticity is a fundamental concept in organic chemistry, referring to the unique stability and reactivity exhibited by certain cyclic compounds. Aromatic compounds, such as benzene, possess a specific arrangement of π-electrons, leading to enhanced stability and distinct properties. The concept of aromaticity extends beyond benzene and encompasses a wide range of compounds, including heterocyclic aromatics and polycyclic aromatic hydrocarbons. Understanding aromaticity is crucial in various areas of organic chemistry and provides insights into the reactivity and behavior of aromatic compounds.