Reactivity and Reactions of Aromatic Compounds: Electrophilic Aromatic Substitution
Aromatic compounds, known for their stability and unique electronic structure, exhibit distinct reactivity patterns compared to other organic compounds. Understanding the reactivity of aromatic compounds is essential in organic chemistry as it enables the prediction and control of their chemical transformations. In this lesson, we will explore the reactivity and various reactions of aromatic compounds, shedding light on important concepts and mechanisms. This lesson will focus on Electrophilic Aromatic Substitution.
Electrophilic Aromatic Substitution (EAS)
The most common reaction type for aromatic compounds is electrophilic aromatic substitution (EAS). Electrophilic aromatic substitution (EAS) is a fundamental reaction type in organic chemistry that involves the substitution of an aromatic hydrogen atom with an electrophile, resulting in the formation of a new functional group on the aromatic ring. EAS reactions are crucial for the introduction of diverse substituents into aromatic compounds, allowing chemists to modify their properties and tune their reactivity.
The mechanism of EAS involves several key steps. Initially, the electrophile interacts with the π-electron system of the aromatic ring, forming a resonance-stabilized intermediate known as the arenium ion or sigma complex. This intermediate involves the formation of a carbon-carbon bond between the electrophile and the aromatic ring. The arenium ion is often stabilized by resonance, delocalizing the positive charge across the ring.
Next, a proton from the arenium ion is abstracted by a base or another nucleophile, leading to the restoration of aromaticity and the formation of the final substituted aromatic compound. The removal of the proton is critical for the completion of the substitution reaction.
The regioselectivity of EAS reactions is determined by the relative reactivity of the aromatic ring positions. Electron-donating groups, such as alkyl groups or methoxy groups, enhance the electron density on the ring and increase the reactivity at the ortho and para positions. Conversely, electron-withdrawing groups, such as nitro or carbonyl groups, decrease the electron density and direct the electrophile to the meta position.
Lets take a closer look using Bromination as an example:
Attack of Bromine on Aluminum moiety
First, a Br2 molecule reacts with an AlBr3 molecule to form a Br2-AlBr3 molecule:
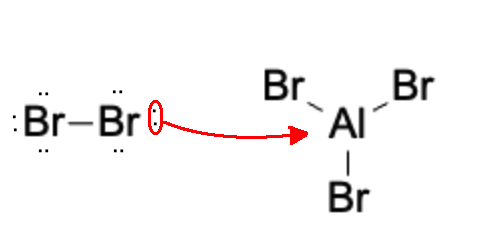
Next, a Br molecule detaches, forming a Br+ anion which will act as our electrophile.
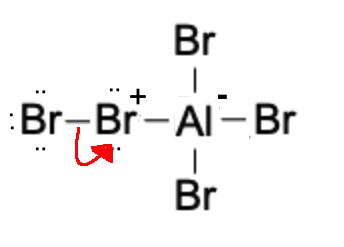
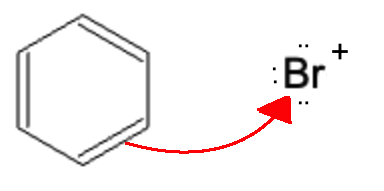
The Br+ electrophile is then attacked by the benzene nucleophile.
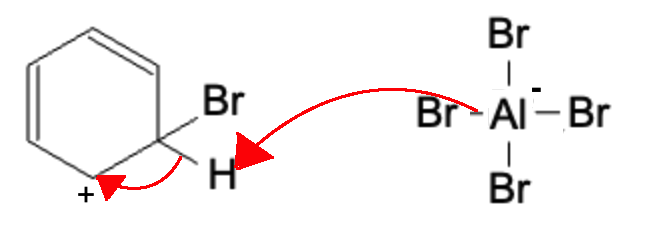
A Br from the AlBr4- molecule generate earlier attacks the hydrogen atom, causing the electrons from that bond to be sent over to the carbocation, forming the finalized product.
Final Product
Reactions to Substituted Benzenes - Ortho, Meta, Para directing groups
When the benzene ring is by itself, the reaction proceeds on any of the 6 carbon atoms. But what happens if you are working with a benzene that has a functional group on it? Depending on the functional group, the functional group may be classified as "ortho, para directing" or as "meta" directing. These terms have to do with the position on the benzene ring:
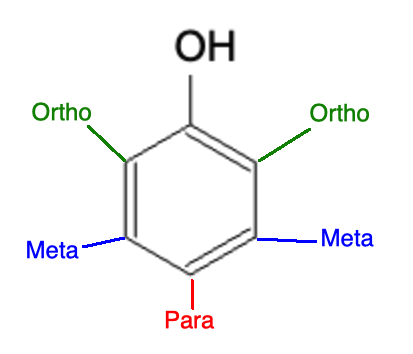
Below is a list of some of the common ortho/para directing groups:

Below is a list of some of the common meta directing groups:

Summary
In summary, electrophilic aromatic substitution (EAS) is a class of reactions where an electrophile substitutes a hydrogen atom in an aromatic ring. The reactivity and regioselectivity of EAS reactions are influenced by the presence of directing groups on the aromatic ring. These directing groups can be broadly classified into ortho/para directing groups and meta directing groups based on their effect on the regioselectivity of the reaction.
Ortho/para directing groups, such as alkyl groups and most activating substituents, donate electron density to the ring through resonance or inductive effects. This increases the electron density at the ortho and para positions, making these positions more reactive and directing the incoming electrophile to these sites.
On the other hand, meta directing groups, including electron-withdrawing substituents like nitro and carbonyl groups, withdraw electron density from the ring. This decreases the electron density at the ortho and para positions, rendering them less reactive. Consequently, the electrophile is directed to the meta position, which has relatively higher electron density due to the presence of the meta directing group.
It is important to note that the directing effects of these groups can be influenced by factors such as steric hindrance, neighboring substituents, and reaction conditions. Additionally, some groups can exhibit dual directing effects depending on the specific circumstances.
Understanding the directing effects of various groups is crucial in predicting the regioselectivity of EAS reactions. By considering the electronic and steric factors associated with the directing groups, organic chemists can make informed predictions about the outcome of these reactions, allowing for the synthesis of a wide range of aromatic compounds with desired substitution patterns.
Test Your Knowledge:
What are 5 meta directing functional groups?
What is the mechanism for a standard EAS reaction?