Introduction to Organometallic Compounds
Organometallic compounds are fascinating molecules that bridge the realms of organic and inorganic chemistry. Their unique characteristics and reactivity make them indispensable tools in modern organic synthesis. In this lesson, we will explore the world of organometallic compounds, understand their definition, importance, and review their bonding and reactions.
Definition and Characteristics of Organometallic Compounds:
Organometallic compounds are compounds that feature a direct bond between a metal atom and one or more carbon atoms. This distinctive bond imparts a range of properties and reactivities to these compounds. Organometallic compounds are typically highly reactive, exhibit diverse bonding patterns, and can act as either nucleophiles or electrophiles in chemical reactions.
Importance and Applications in Organic Synthesis:
The significance of organometallic compounds in organic synthesis cannot be overstated. They serve as powerful reagents for constructing carbon-carbon bonds, facilitating transformations that were once considered challenging or impossible. Through reactions such as cross-coupling and carbonyl additions, organometallic compounds enable the synthesis of complex organic molecules with high efficiency and selectivity.
Overview of Common Organometallic Reagents and Catalysts:
Several organometallic reagents and catalysts find extensive use in organic chemistry. For instance, Grignard reagents and organolithium compounds are widely employed nucleophilic reagents, known for their ability to form carbon-carbon bonds. Transition metal complexes, such as Wilkinson's catalyst and Grubbs' catalyst, act as catalysts in various transformations, enabling new synthetic pathways and enhancing reaction efficiency.
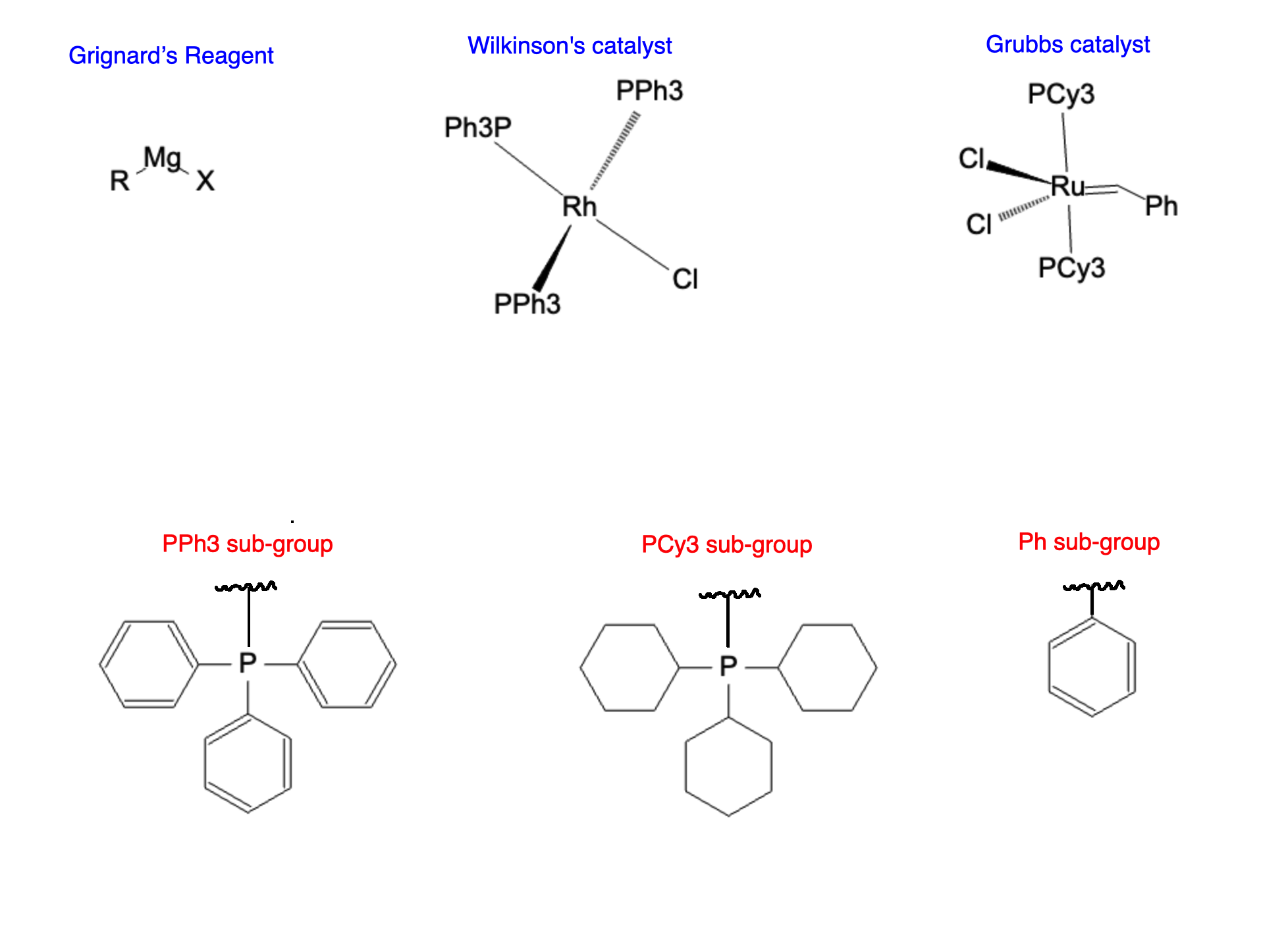
Grignard reagents have a magnesium (Mg) center while being attached to an carbon chain (R) and a halogen (X). Wilkinson's catalyst, featuring a rhodium (Rh) metal center, is renowned for its role in catalyzing hydrogenation reactions. Grubbs' catalyst, based on a ruthenium complex (Ru), revolutionized the field of olefin metathesis.
Bonding in Organometallic Compounds:
Understanding the bonding in organometallic compounds is key to unraveling their reactivity. Coordination complexes and ligands play a crucial role in stabilizing these compounds. The metal-carbon bond involves both sigma and pi bonding, where electrons are shared between the metal and carbon atoms. This bonding interaction dictates the stability and behavior of organometallic compounds.
Organometallic compounds exhibit intriguing reactivity, acting as both nucleophiles and electrophiles. Nucleophilic additions, eliminations, and substitutions are common reactions. Furthermore, oxidative addition and reductive elimination reactions play significant roles in the transformation of these compounds. The understanding of these reaction mechanisms enables precise control over synthetic pathways.
A wide array of reactions involve organometallic compounds. These reactions include carbon-carbon bond formation, functional group transformations, and metal-mediated reactions. The versatility of organometallic compounds allows for the creation of complex organic structures and the development of new synthetic methodologies.
Summary
Organometallic compounds have revolutionized the field of organic chemistry, providing powerful tools for synthetic chemists. In this article, we explored the definition and characteristics of organometallic compounds, their importance in organic synthesis, bonding patterns, examples of common complexes, and reactivity in various reactions. By understanding the unique properties of these compounds, we unlock the ability to form new synthetic pathways that were otherwise unachievable in the field of organic chemistry.