Transition Metal Catalysis and Coupling Reagents
Grubbs and Wilkinson catalysts are two remarkable examples of transition metal catalysts that have revolutionized the field of organic chemistry, particularly in the area of olefin metathesis reactions. Developed by Robert H. Grubbs and Geoffrey Wilkinson, respectively, these catalysts have enabled the efficient formation and transformation of carbon-carbon double bonds, offering new avenues for the synthesis of complex organic molecules. In this article, we will explore the Grubbs and Wilkinson catalysts, their mechanisms, and their impact on the field of organic synthesis.
Grubbs Catalysts:
Grubbs catalysts are a class of ruthenium-based catalysts that are widely employed in olefin metathesis reactions. They were developed by Robert H. Grubbs and his research team, who received the Nobel Prize in Chemistry in 2005 for their contributions to the development of this catalyst system. Grubbs catalysts are typically composed of a ruthenium center coordinated with a N-heterocyclic carbene ligand. These catalysts exhibit remarkable selectivity and tolerance toward various functional groups, making them valuable tools in the synthesis of complex organic molecules.
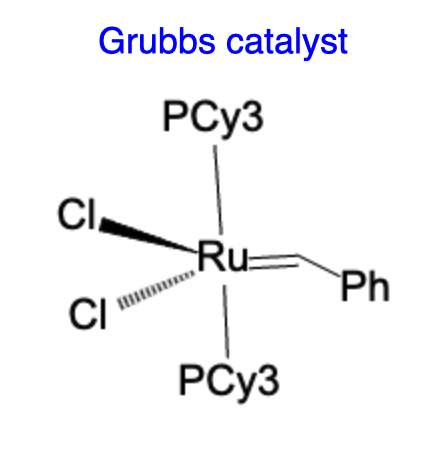
The mechanism of olefin metathesis using Grubbs catalysts involves the coordination of the olefin substrate to the ruthenium center, followed by the formation of a metallacyclobutane intermediate. This intermediate undergoes a series of intramolecular rearrangements and exchange reactions, leading to the desired olefin metathesis products. Grubbs catalysts have been successfully employed in a wide range of applications, including the synthesis of natural products, polymers, and pharmaceutical compounds.
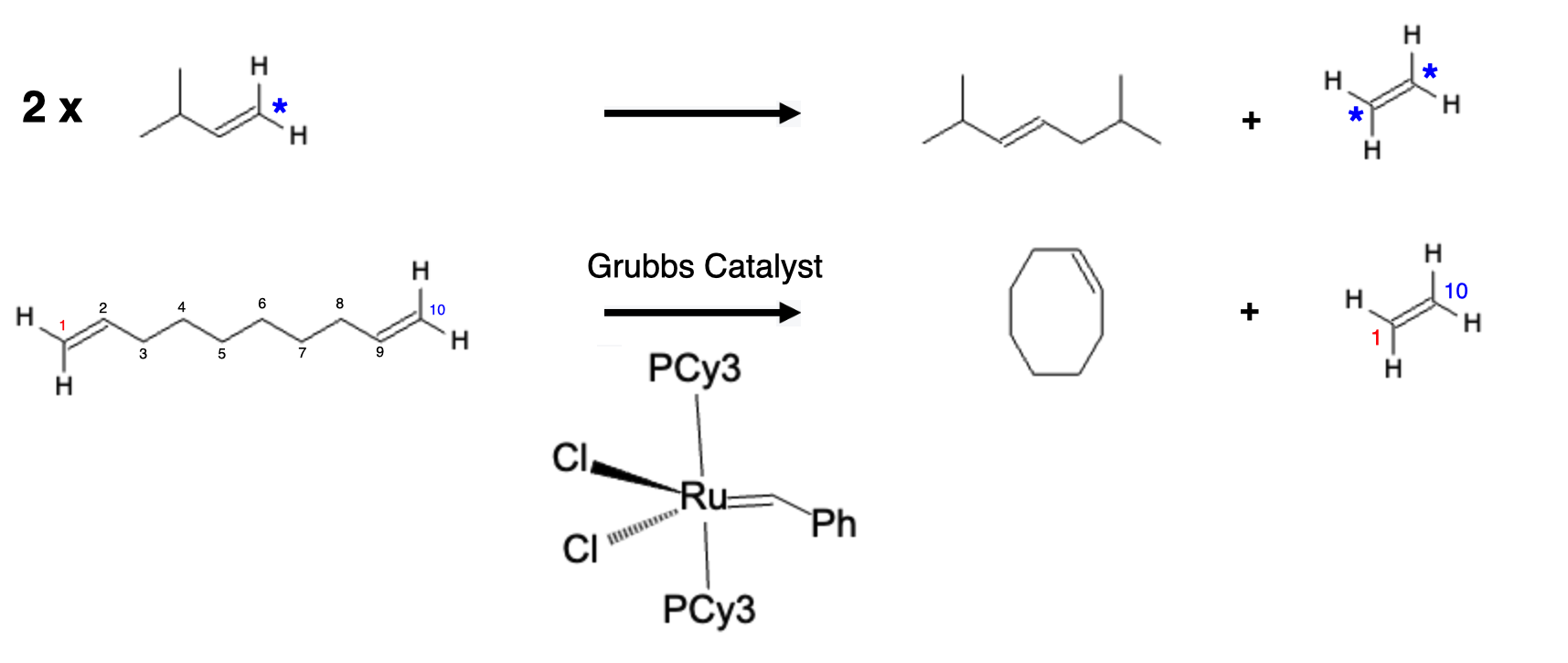
Grubbs Catalysts are used to combine two alkenes together; this is often represented by two distinct terminal alkenes or a long alkene chain that has two terminal alkenes that can be bound together to form a ring stucture:
Wilkinson Catalyst:
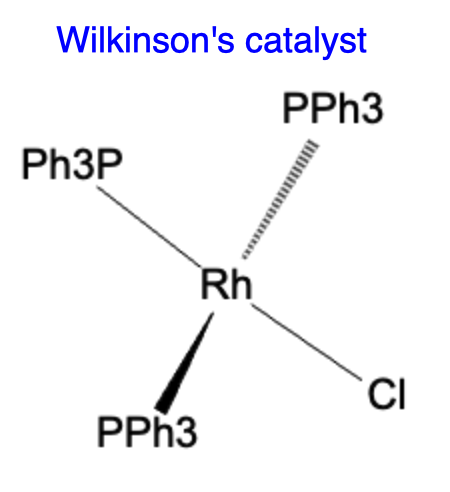
Wilkinson catalyst, also known as Wilkinson's rhodium complex, is a rhodium-based catalyst discovered by Geoffrey Wilkinson. It consists of a rhodium center coordinated with three triphenylphosphine (P(Ph)3) ligands and a chloride ligand. The Wilkinson catalyst is highly effective in various catalytic processes, including hydrogenation and hydroformylation reactions. It has played a significant role in the development of catalytic methodologies and has been extensively employed in industrial processes.
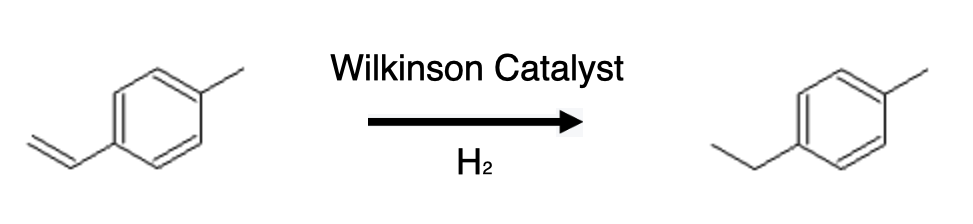
In hydrogenation reactions, the Wilkinson catalyst promotes the addition of hydrogen to unsaturated compounds, resulting in the formation of saturated products. The catalytic cycle involves the coordination of the substrate to the rhodium center, followed by the activation of hydrogen and its subsequent addition to the coordinated substrate. The Wilkinson catalyst exhibits excellent activity and selectivity, making it a valuable tool in the synthesis of a wide range of organic compounds.
Coupling Reactions (Palladium Reagents)
Suzuki and Heck reactions are two prominent coupling reactions widely utilized in organic synthesis for the formation of carbon-carbon bonds. These reactions involve the coupling of an organic halide or pseudo-halide with an organoboron or an olefin, respectively. The coupling reagents employed in these reactions, known as Suzuki and Heck reagents, play a crucial role in facilitating the bond formation process. In this article, we will delve into the details of Suzuki and Heck reagents, their mechanisms, and their applications in organic chemistry.
Suzuki Reaction:
The Suzuki reaction, discovered by Akira Suzuki, relies on the use of palladium-catalyzed cross-coupling between an aryl or vinyl halide and an organoboron compound. The key component in the Suzuki reaction is the Suzuki reagent, which is an organoboron compound bearing an aryl or vinyl group and a boronate ester functionality. These reagents are typically prepared by the reaction of aryl or vinyl boronic acids or boronate esters with an appropriate palladium catalyst, often a palladium(II) complex.
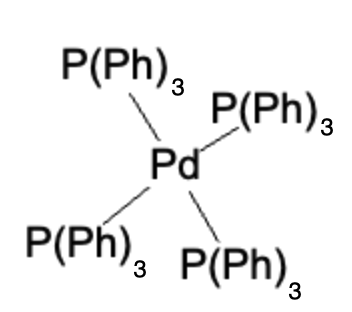
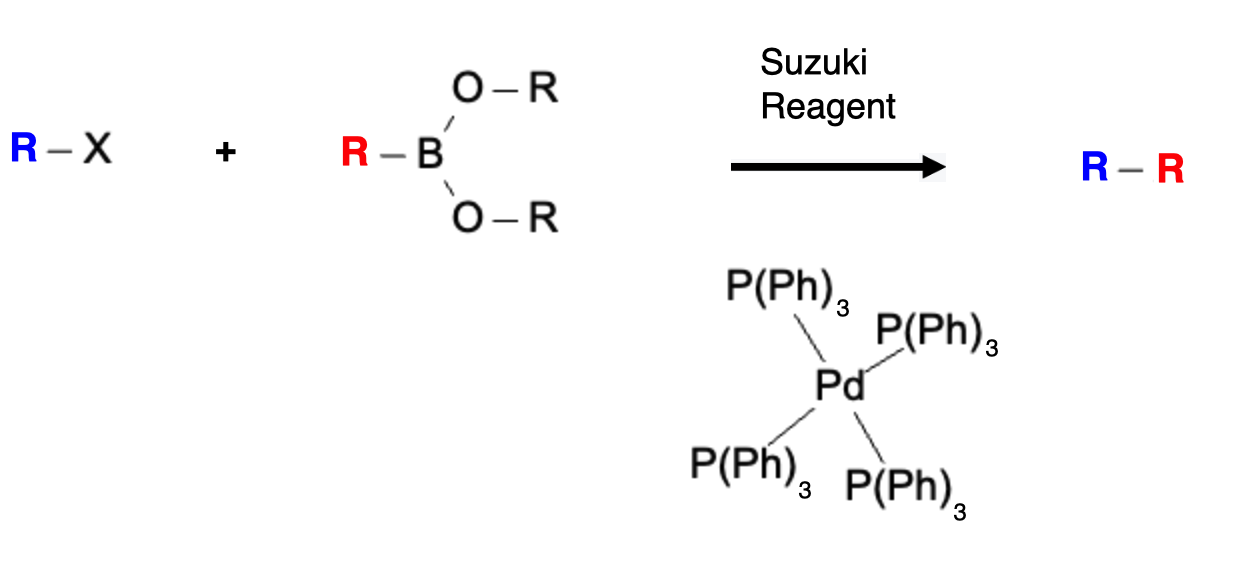
The palladium catalyst facilitates the transmetalation of the boronate ester with the aryl or vinyl halide, followed by reductive elimination, resulting in the formation of the desired coupled product. The Suzuki Reaction follows this general formula:
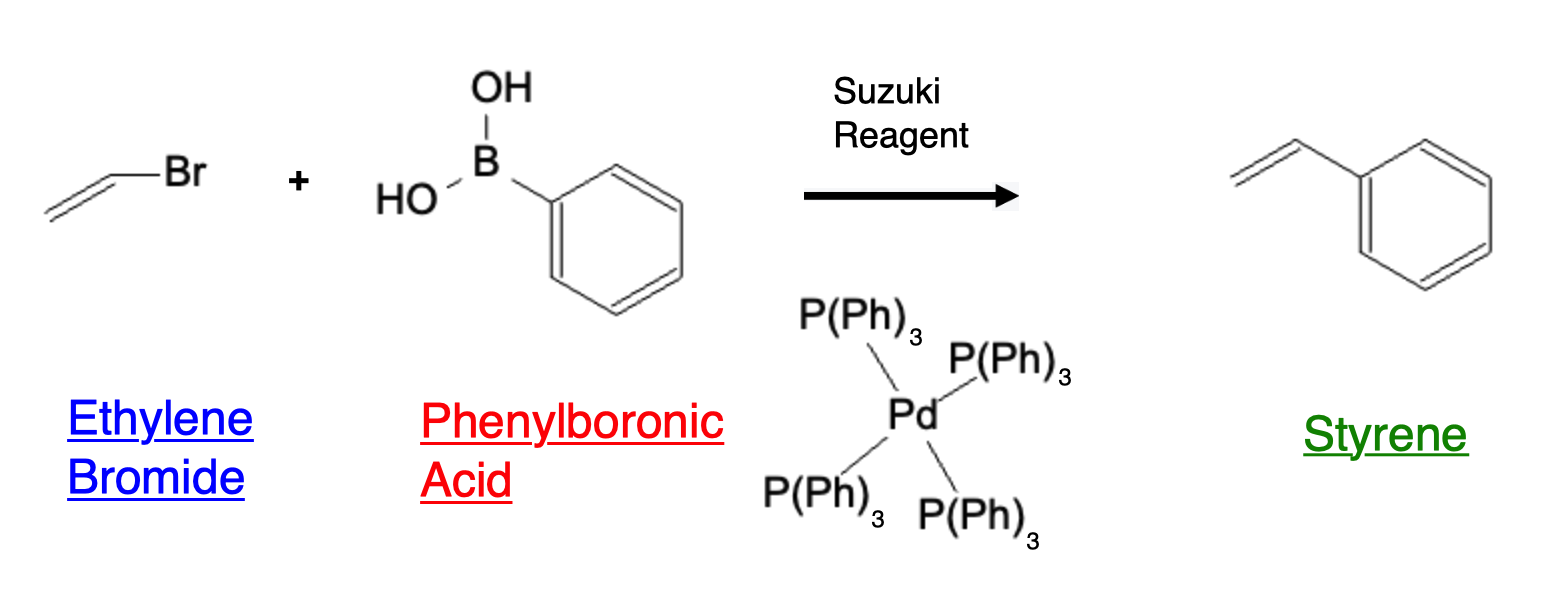
A more specific example is ethylene bromide reacting with phenylboronic acid to form styrene:
Heck Reaction:
The Heck reaction, developed by Richard F. Heck, involves the coupling of an organic halide with an olefin in the presence of a palladium catalyst. Heck reagents, also known as vinyl or aryl halides, serve as the electrophilic partners in this reaction. These reagents are typically prepared by halogenation of the corresponding organic substrate or by other synthetic methods. The reaction proceeds through the coordination of the olefin to the palladium catalyst, followed by migratory insertion of the aryl or vinyl group into the palladium-carbon bond. The reaction is completed by β-hydride elimination, leading to the formation of the coupled product.
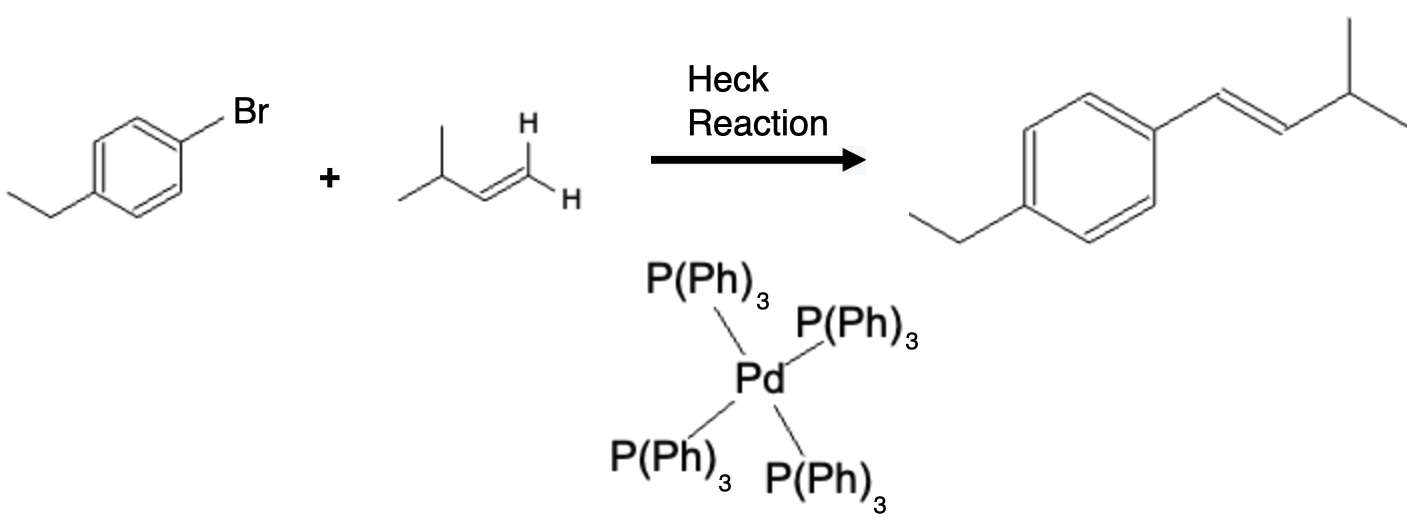
Using a similar reagent as the Suzuki reaction, the Heck reaction also involves a palladium (Pd) catalyst but does not need a boronic acid to complete the reaction. This reaction essentially couples the two reactants together by removing the alkyl (X) group from the alkyl halide and a hydrogen (H) from the alkene:
Summary
Transition metal catalysts play a crucial role in organic chemistry by facilitating a wide range of important reactions. These catalysts, typically composed of transition metal complexes, exhibit unique reactivity and selectivity, making them valuable tools for synthetic chemists. They are capable of activating and transforming organic molecules through various mechanisms, including coordination, electron transfer, and bond activation. Transition metal catalysts find applications in numerous fields, such as pharmaceuticals, materials science, and fine chemical synthesis.
Test Your Knowledge
- What is the difference in uses between the Grubbs catalyst and the Wilkonson catalyst?
- What two types of reactants are needed for the Suzuki reaction? And for the Heck reaction?