Aldol Condensation and Claisen Condensation
Aldol condensation and Claisen condensation are two important carbon-carbon bond-forming reactions in organic chemistry. These reactions play a significant role in the synthesis of complex organic molecules by enabling the formation of new carbon-carbon bonds and the introduction of functional groups. The aldol condensation involves the condensation of an enol or an enolate with an aldehyde or a ketone, leading to the formation of β-hydroxy carbonyl compounds. On the other hand, the Claisen condensation involves the condensation of two ester molecules or an ester with a ketone, resulting in the formation of β-diketone compounds. Both of these reactions have broad applications in organic synthesis and are essential tools for chemists working in various fields, including pharmaceuticals, materials science, and natural product synthesis. In this lesson, we will explore the mechanisms, reaction conditions, and applications of aldol condensation and Claisen condensation, providing a comprehensive overview of these valuable synthetic methods.
Aldol Condensation:
The aldol condensation is a powerful carbon-carbon bond-forming reaction that allows the synthesis of β-hydroxy carbonyl compounds. It involves the condensation of an enol or an enolate with an aldehyde or a ketone, leading to the formation of a new carbon-carbon bond. The reaction proceeds through a nucleophilic addition followed by an elimination of water.
The mechanism of the aldol condensation involves the formation of an enolate ion or enol tautomer of the carbonyl compound, which acts as the nucleophile. The nucleophile attacks the electrophilic carbonyl carbon of another carbonyl compound, resulting in the formation of a β-hydroxy carbonyl compound. The reaction conditions for aldol condensation typically involve the use of a base to generate the enolate or enol intermediate. Common bases used include hydroxides, alkoxides, and amines. The reaction can be conducted in both aqueous and non-aqueous solvents, depending on the reactants and desired conditions.
Let's take a simple example starting with acetone. The first step involves enolate formation using a strong base:
Enolate formation
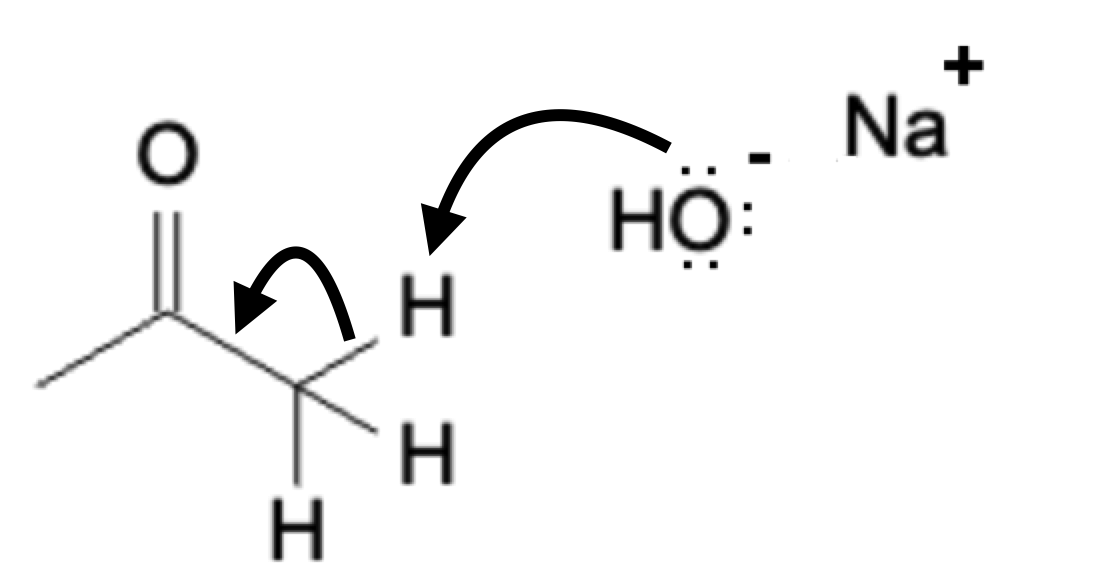
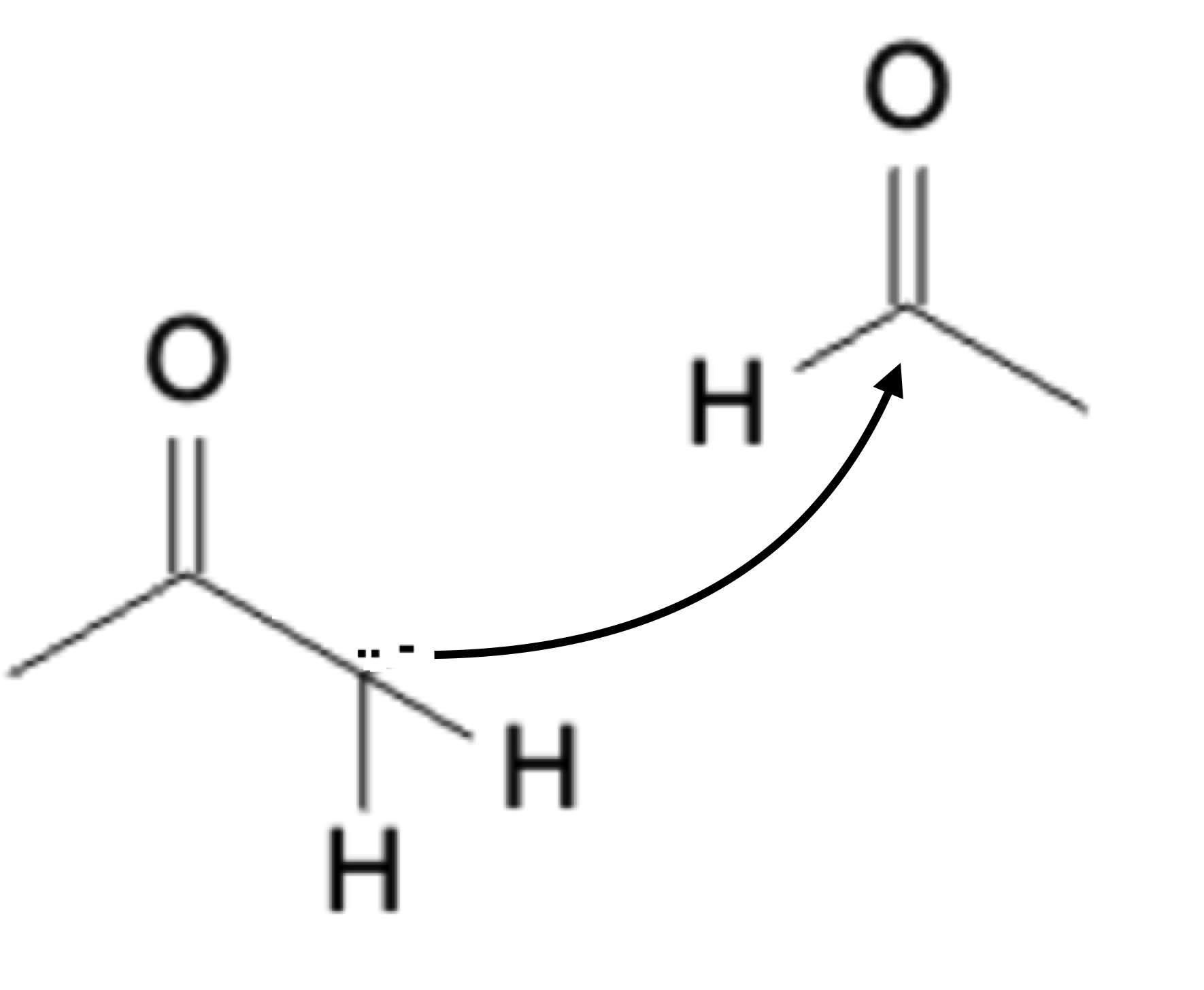
Once the enolate is formed, it becomes a strong nucleophile and seeks out the electrophilic center of another carbonyl group:
Addition
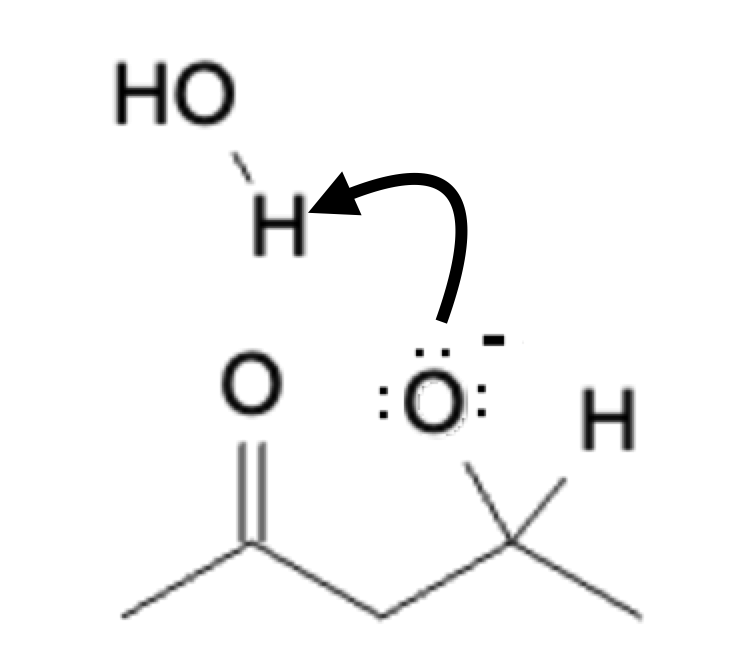
Next, a water molecule protonates the negatively charged oxygen:
Hydration
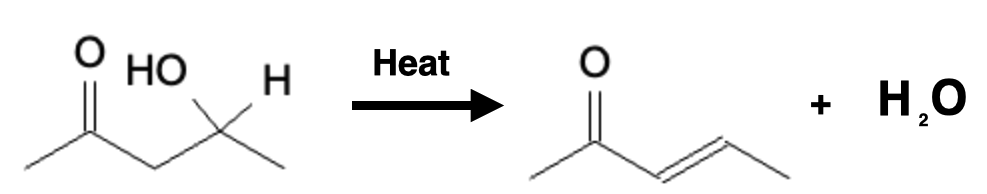
Once the addition product has been hydrated, the addition product can transform into the condensation product with the addition of heat:
Condensation
There are several types of aldol condensations. Crossed aldol condensation involves the reaction between different carbonyl compounds. Intramolecular aldol condensation occurs when a single molecule contains both the carbonyl and nucleophilic moieties necessary for the reaction. Directed aldol condensation involves the use of specific reagents or catalysts to control the regioselectivity and stereoselectivity of the reaction.
The aldol condensation is widely utilized in organic synthesis due to its versatility and ability to create complex molecules. It is particularly useful in the synthesis of natural products, pharmaceuticals, and other important organic compounds.
Claisen Condensation:
The Claisen condensation is another important carbon-carbon bond-forming reaction that involves the condensation of two ester molecules or an ester with a ketone. It leads to the formation of a β-diketone compound. Similar to the aldol condensation, the Claisen condensation proceeds through a nucleophilic addition and elimination of a leaving group, which in this case is an alkoxide ion.
The mechanism of the Claisen condensation involves the formation of an enolate intermediate from one of the ester substrates. The enolate then attacks the electrophilic carbonyl carbon of another ester or ketone, resulting in the formation of a β-diketone compound. The reaction conditions typically involve the use of a base to generate the enolate intermediate. Common bases used in Claisen condensation include alkoxides and amines. The reaction is typically conducted in non-aqueous solvents to ensure the stability of the reagents.

Claisen condensation can occur both intramolecularly and intermolecularly. In intramolecular Claisen condensation, a single molecule contains two ester groups that undergo condensation, leading to the formation of a cyclic β-diketone compound. Intermolecular Claisen condensation involves the reaction between two separate ester molecules.

The Claisen condensation is widely utilized in organic synthesis, particularly for the synthesis of β-diketone compounds. These compounds serve as important intermediates in the synthesis of various organic molecules, including natural products, pharmaceuticals, and complex organic materials.
Summary
Both the aldol condensation and Claisen condensation are powerful carbon-carbon bond-forming reactions. The aldol condensation allows for the synthesis of β-hydroxy carbonyl compounds, while the Claisen condensation leads to the formation of β-diketone compounds. These reactions find broad applications in organic synthesis, enabling the construction of complex molecules and facilitating the synthesis of natural products and pharmaceuticals.
Test Your Knowledge
- Describe the mechanism of the aldol condensation and the key reaction conditions involved.
- Differentiate between intramolecular and intermolecular Claisen condensation reactions, providing an example of each.