Formation and Reactivity of Enolates
Enolates are resonance-stabilized anions that play a crucial role in organic chemistry as versatile nucleophiles. Understanding their formation, reactions, and applications is essential for mastering the field of organic synthesis. An enolate is the conjugate base of an enol.
Formation of Enolates:
Enolates are formed by deprotonation of α-carbons adjacent to carbonyl groups. This deprotonation can occur through various methods. One common approach is the use of strong bases, such as alkoxides or amides, which abstract the α-hydrogen, generating the enolate anion. Another method involves the use of acidic conditions, where the α-hydrogen is protonated by an acid, followed by deprotonation by a base. The presence of an acidic α-hydrogen is crucial for enolate formation.
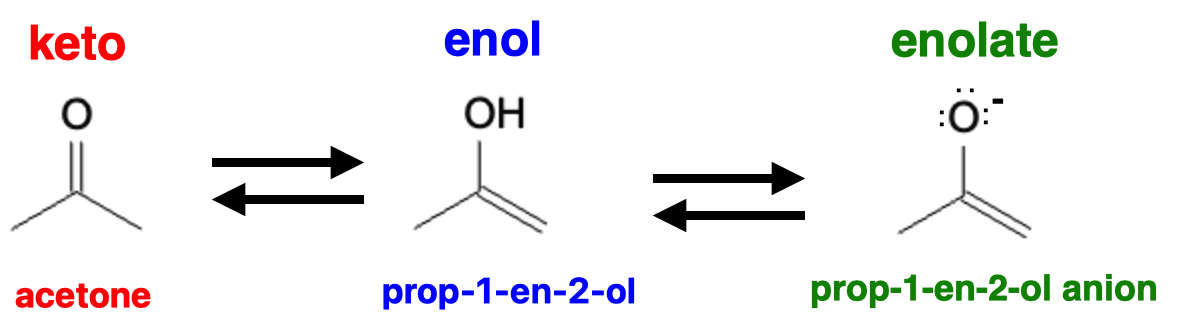
Reactions of Enolates:
Enolates exhibit nucleophilic reactivity, making them excellent candidates for nucleophilic addition reactions. They readily react with electrophilic carbonyl compounds, such as aldehydes, ketones, and esters. The nucleophilic attack of the enolate on the electrophilic carbon of the carbonyl group leads to the formation of new carbon-carbon bonds. This reaction is known as nucleophilic addition. Enolates also participate in other reactions, including condensation reactions like the aldol condensation and Claisen condensation, as well as the Michael addition.
Enolates are invaluable tools in organic synthesis due to their synthetic versatility. The ability of enolates to form new carbon-carbon bonds makes them essential for the construction of complex molecules. They are particularly useful in the synthesis of natural products and pharmaceuticals. Enolates are also utilized in retrosynthetic analysis, allowing chemists to plan multistep syntheses by strategically utilizing enolates as intermediates. Additionally, enolates find applications in the protection and deprotection of functional groups, facilitating selective reactions in complex synthetic sequences.
Understanding the formation and reactivity of enolates provides organic chemists with powerful tools for designing and executing synthetic strategies. Their nucleophilic nature and the ability to form new carbon-carbon bonds make them indispensable in the construction of complex molecules. By harnessing the reactivity of enolates, chemists can access a wide range of reactions and achieve significant advancements in organic synthesis.
Summary
Enolates are resonance-stabilized anions formed through deprotonation of α-carbons adjacent to carbonyl groups. They exhibit nucleophilic reactivity and can undergo nucleophilic addition reactions with electrophilic carbonyl compounds. Enolates are essential in various synthetic reactions, including condensations, and find extensive applications in natural product synthesis and retrosynthetic analysis.
Test Your Knowledge:
How are enolates formed, and what are the different methods for generating enolates?