Introduction to Enols
Enols are organic compounds that contain a hydroxyl group (-OH) attached to a carbon-carbon double bond (C=C). They are tautomeric forms of carbonyl compounds, meaning they can exist in equilibrium with their keto counterparts. This equilibrium is referred to as tautomerization.
Tautomerization involves the migration of a proton (H) from the α-carbon of the carbonyl group to the oxygen atom, resulting in the formation of an enol or keto form. The equilibrium between the keto and enol forms can be influenced by various factors, including temperature, solvent, and substituent effects.
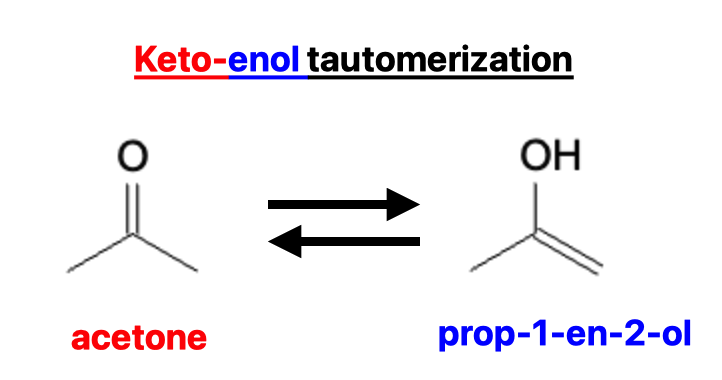
Take the example of acetone and prop-1-en-2-ol below, which represents the simplest tautomerization:
Stability of Enols
The stability of enols depends on several factors. Resonance stabilization plays a significant role, as enols can exhibit resonance structures that delocalize the negative charge on the oxygen atom. Intramolecular hydrogen bonding is another stabilizing factor, where enols can form hydrogen bonds between the hydroxyl group and a neighboring carbonyl group. Substituent effects also come into play, as electron-withdrawing groups adjacent to the double bond can stabilize enols by withdrawing electron density from the hydroxyl group.
Resonance example:
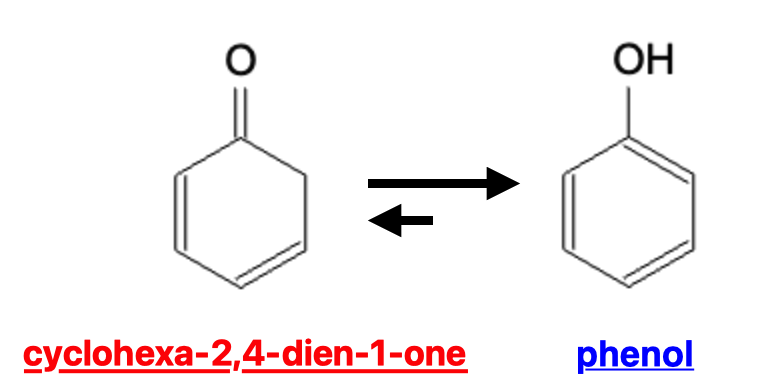
A common example of an enol being more stable than the keto counter part via resonance stabilization is seen with cyclohexa-2,4-dien-1-one vs phenol:
Hydrogen Bonding example:
An example of the enol being more stable than the keto form due to hydrogen bonding is pentane-2,4-dione vs (E)-4-hydroxypent-3-en-2-one:

Substituent Effects example:
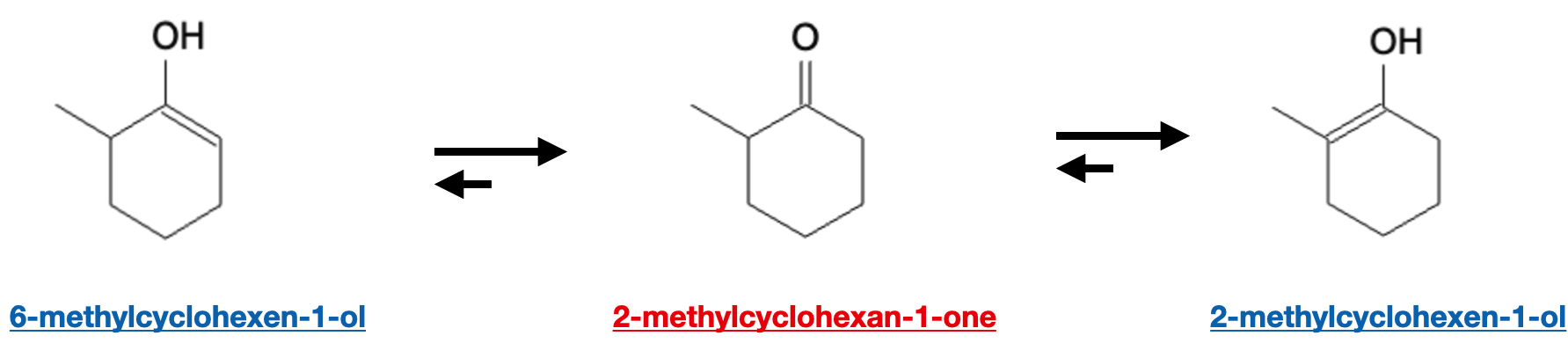
An example of the enol being more stable than the keto form due to substituent effects is 6-methylcyclohexen-1-ol vs 2-methylcyclohexan-1-one vs 2-methylcyclohexen-1-ol where 2-methylcyclohexen-1-ol is the most stable due to the double bond being located between the substituent group and the alcohol group:
Keto form is electrophilic, Enol form is nucleophilic
In the tautomeric equilibrium between the keto and enol forms, the keto form is generally more stable due to the electron-withdrawing nature of the carbonyl group. The presence of the electronegative oxygen atom pulls electron density away from the adjacent carbon atom, making it electron-deficient or electrophilic. As a result, the keto form is more prone to react with nucleophiles.
On the other hand, the enol form possesses a nucleophilic character due to the presence of the electron-rich hydroxyl (-OH) group attached to the carbon-carbon double bond. The oxygen atom in the enol form has a lone pair of electrons that can readily participate in nucleophilic reactions. This nucleophilic nature arises from the higher electron density around the hydroxyl group, making it more reactive towards electrophiles.
The electrophilic nature of the keto form and the nucleophilic nature of the enol form play significant roles in various organic reactions, such as nucleophilic addition, condensation, and substitution reactions. Understanding the electrophilic and nucleophilic characteristics of the keto and enol forms is crucial for predicting the reactivity and outcomes of these reactions.
Summary
Enols are organic compounds that contain a hydroxyl group attached to a carbon-carbon double bond. They exist in equilibrium with their keto counterparts and can interconvert through tautomerization. The stability of enols is influenced by factors such as resonance stabilization, intramolecular hydrogen bonding, and substituent effects. Understanding the concept of enols and their equilibrium with keto forms is essential for predicting their reactivity and the outcomes of related reactions.
Test your knowledge
- What factors can influence the stability of enols?
- Provide an example of an enolizable compound and explain how it can undergo tautomeric equilibrium with its keto form.