Introduction to Carbohydrates
In this lesson, we will be discussing the structure, classification, and properties of carbohydrates. Understaning these concepts is fundamental to comprehending the intricate roles they play in biological systems. Carbohydrates serve as a primary source of energy, contribute to cellular structures, and participate in various biochemical processes. Through this lesson, we will delve into the fundamental concepts of carbohydrates, including their definition, classification based on size, and an overview of common monosaccharides.
Definition and General Characteristics of Carbohydrates:
Carbohydrates, also known as saccharides, are organic compounds consisting of carbon, hydrogen, and oxygen atoms. They are the most abundant class of biomolecules and serve as a crucial source of energy in living organisms. Carbohydrates are characterized by their empirical formula, (CH2O)n, where n represents the number of carbon atoms. They exhibit a wide range of structural diversity, from simple sugars to complex polysaccharides.
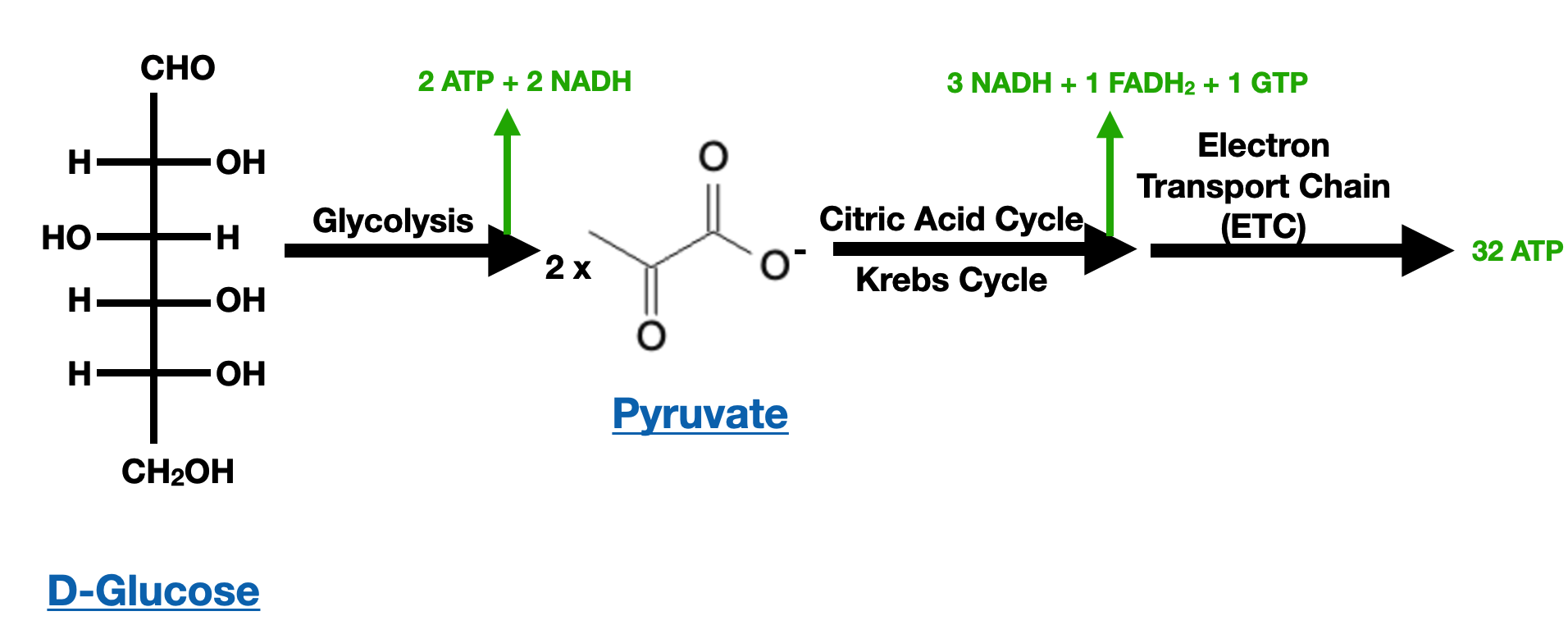
One of the defining features of carbohydrates is their role as a primary energy source in cells. Through cellular respiration, carbohydrates are broken down to release energy in the form of adenosine triphosphate (ATP), which fuels various metabolic processes. Additionally, carbohydrates have important structural functions, serving as building blocks for components like cell walls, extracellular matrices, and connective tissues.
Carbohydrates exhibit remarkable structural diversity, which contributes to their wide range of biological functions. They can exist as monosaccharides, which are single sugar units, or as larger molecules formed by the combination of multiple monosaccharide units. Monosaccharides are further classified based on the number of carbon atoms they contain, with common examples including trioses, tetroses, pentoses, and hexoses.
Furthermore, carbohydrates can form glycosidic bonds through condensation reactions between hydroxyl groups of two monosaccharide molecules. These bonds result in the formation of disaccharides, such as sucrose and lactose, or more complex polysaccharides like starch, cellulose, and glycogen.
Importance of Carbohydrates: Energy (ATP) Generation from a single Glucose molecule
Classification of Carbohydrates Based on Size:
Carbohydrates are classified into three main groups based on their size: monosaccharides, disaccharides, and polysaccharides. Monosaccharides are the simplest form of carbohydrates and cannot be further hydrolyzed to yield smaller carbohydrate units. Examples of monosaccharides include glucose, fructose, and galactose. Disaccharides are formed by the condensation reaction between two monosaccharide units, resulting in the formation of a glycosidic bond. Common disaccharides include sucrose, lactose, and maltose. Polysaccharides, such as cellulose, starch, and glycogen, are composed of long chains of monosaccharide units and serve as storage or structural materials in living organisms.
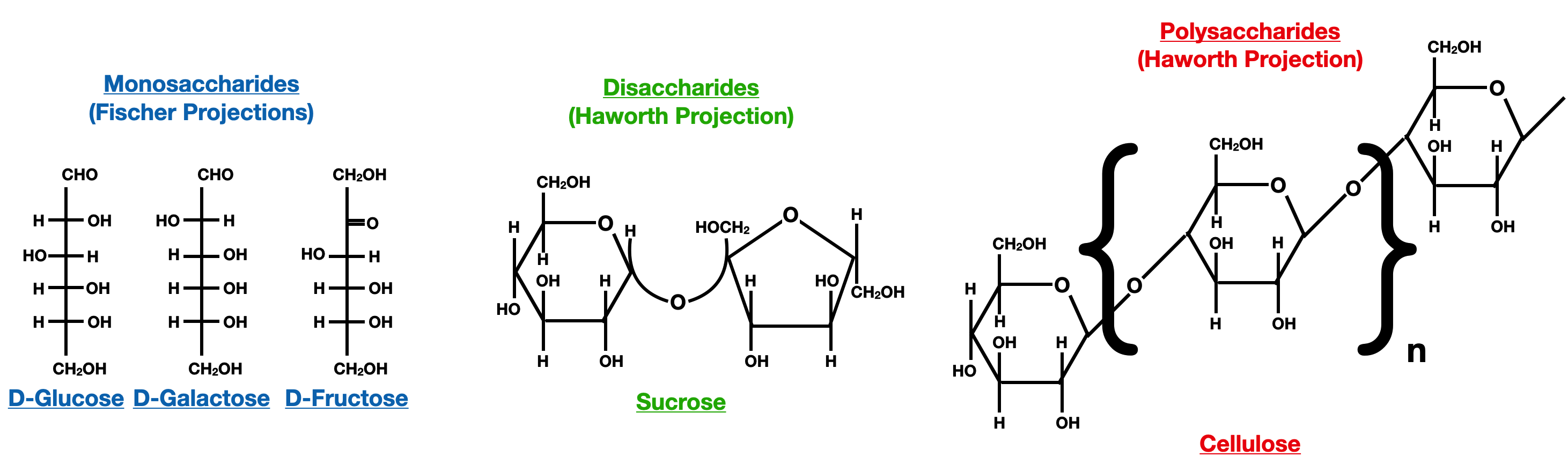
Overview of Common Monosaccharides:
Glucose is a hexose monosaccharide and is the primary energy source for most organisms. It exists in two forms: D-glucose and L-glucose, which are enantiomers. D-glucose is the naturally occurring form and is commonly referred to as "blood sugar."
Fructose is a ketose monosaccharide and is found in fruits and honey. It is a common sweetener used in food and beverages.
Galactose is an aldose monosaccharide and is a component of lactose, the sugar found in milk.
Introduction to the Concept of Chirality and Enantiomers in Carbohydrate Molecules:
Chirality is a fundamental concept in organic chemistry that plays a crucial role in the understanding of carbohydrate molecules. Chiral molecules are those that possess a non-superimposable mirror image. In other words, they exhibit handedness, much like our left and right hands. This property arises due to the presence of an asymmetric carbon atom, also known as a stereogenic center or chiral center, which is a carbon atom bonded to four different substituents.
In carbohydrate molecules, chirality is commonly found at the asymmetric carbon(s) within the monosaccharide units. These monosaccharides can exist as two mirror-image forms known as enantiomers. Enantiomers have identical chemical and physical properties except for their interaction with other chiral molecules. They rotate plane-polarized light in opposite directions, either clockwise (dextrorotatory, labeled as "+") or counterclockwise (levorotatory, labeled as "-").
The presence of chiral centers in carbohydrate molecules gives rise to the phenomenon of optical isomerism or optical activity. Enantiomers are optically active, meaning they can rotate the plane of polarized light. The extent of this rotation is quantified by a property called specific rotation, denoted by the Greek letter alpha (α).
It is important to note that carbohydrates in living systems predominantly exist in the D-configuration, which refers to the configuration of the asymmetric carbon(s) based on the Fischer projection. This convention assigns the hydroxyl group on the chiral carbon farthest from the carbonyl group to the right side. The L-configuration, on the other hand, represents the mirror image of the D-configuration.
In monosaccharides presented in the Fischer projections, the quickest way to determine between the D and L configuration is to locate the position of the bottom hydroxyl group (-OH). In the examples below, the L configurations will have the hydroxyl group on the left and the D configurations will have it on the right.
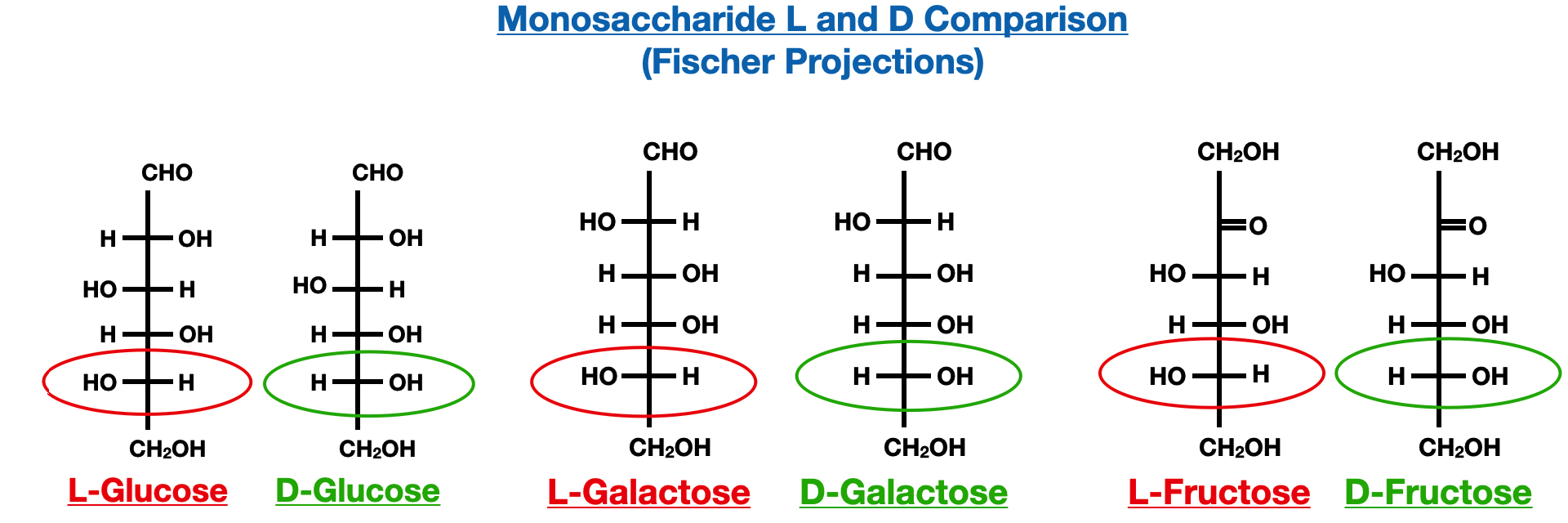
Summary
In this lesson, we have introduced the fundamental concepts of carbohydrates, including their definition, classification based on size, and an overview of common monosaccharides. We have also explored the concept of chirality and enantiomers in carbohydrate molecules. Understanding these foundational aspects of carbohydrates is crucial for delving into more advanced topics related to their structure, reactions, and biological significance.
Test your knowledge
- What is the difference between a monosaccharide, disaccharide, and polysaccharide?
- How can you tell the difference between the D and L configuration of a monosaccharide?