Reactions of Carbohydrates
Carbohydrates, also known as saccharides or sugars, are fundamental biomolecules found in living organisms. They serve as a primary source of energy and play crucial roles in cellular processes, including cell signaling, molecular recognition, and structural support. The unique chemical and structural properties of carbohydrates arise from the diverse functional groups present in their structures. In this lesson, we will explore the different reactions that carbohydrates undergo and the functional groups involved, providing a comprehensive understanding of their reactivity and their significance in both biological systems and organic synthesis.
The reactions of carbohydrates encompass a wide range of transformations, including oxidation, reduction, esterification, acetal formation, glycoside formation, and hydrolysis. These reactions are governed by the functional groups present in carbohydrates, such as hydroxyl groups, aldehyde or ketone groups, and glycosidic bonds. Understanding these reactions and the underlying mechanisms is crucial for elucidating carbohydrate metabolism, studying carbohydrate-based biomolecules, and designing synthetic strategies for carbohydrate synthesis.
Oxidation and Reduction Reactions
Oxidation and reduction reactions play a significant role in the metabolism and transformations of carbohydrates. These reactions involve the gain or loss of electrons, leading to changes in the oxidation state of the carbon atoms within the carbohydrate molecule. Oxidation involves the loss of electrons, while reduction involves the gain of electrons.
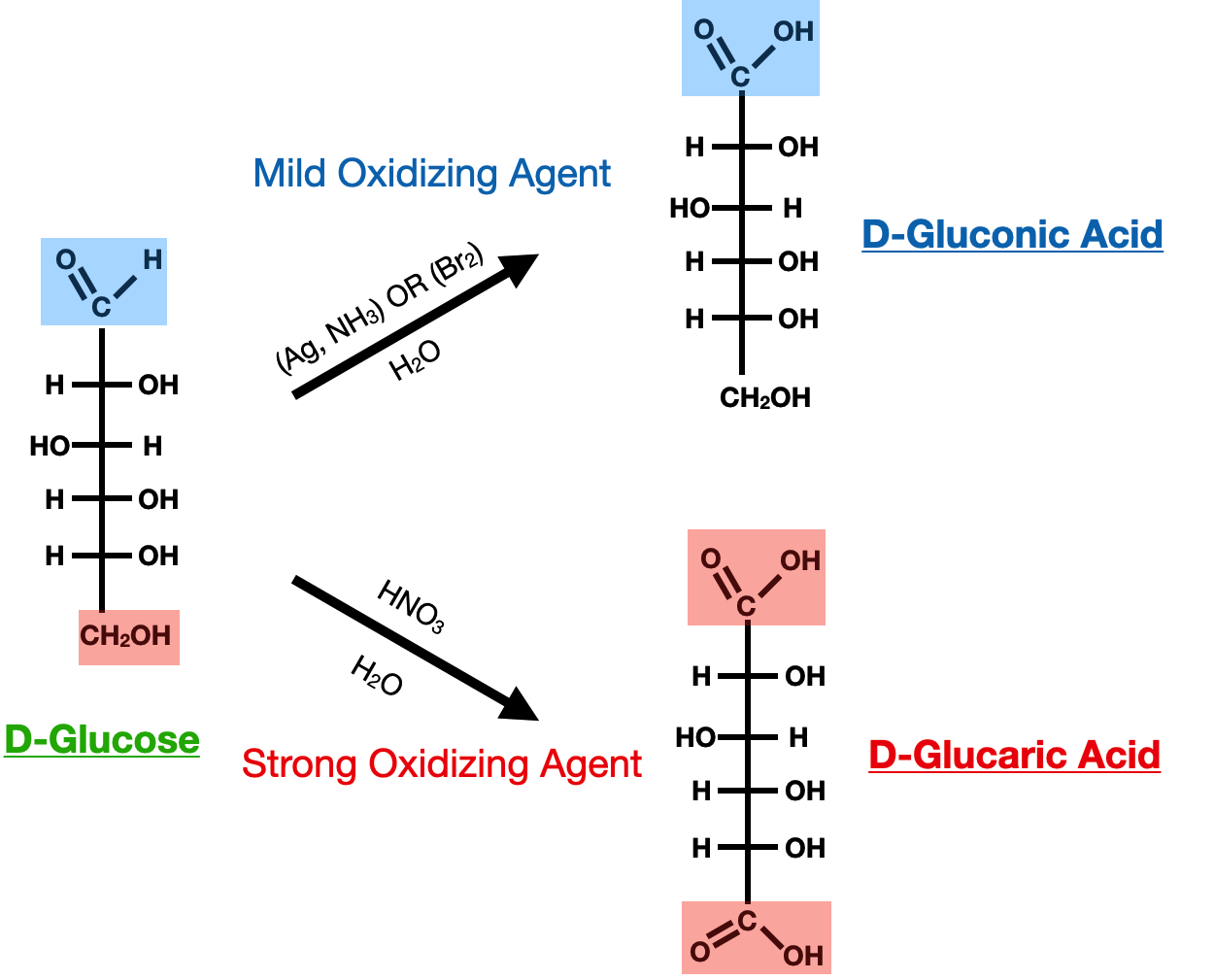
In the context of carbohydrates, oxidation reactions often occur at the aldehyde or terminal carbonyl group of monosaccharides. The aldehyde group can be oxidized to a carboxylic acid, resulting in the formation of an aldonic acid. This oxidation reaction is commonly observed with oxidizing agents such as Tollens' reagent or Benedict's solution. On the other hand, ketose sugars, which contain a ketone group, can undergo oxidation to form a lactone.
Mild oxidizing agents, (such as Tollens' reagent or Br2/H2O) oxidize the aldehyde group (CHO) to a carboxylic acid group (COOH) where as strong oxidizing agents (such as HNO3) oxidize both ends of the sacharride forming carboxylic acids on both ends:
Reduction reactions, on the other hand, involve the addition of electrons to a carbohydrate molecule, resulting in the formation of a reduced form. One common example of a reduction reaction in carbohydrates is the conversion of an aldehyde group to an alcohol. This reaction can be catalyzed by reducing agents such as sodium borohydride (NaBH4) or lithium aluminum hydride (LiAlH4).
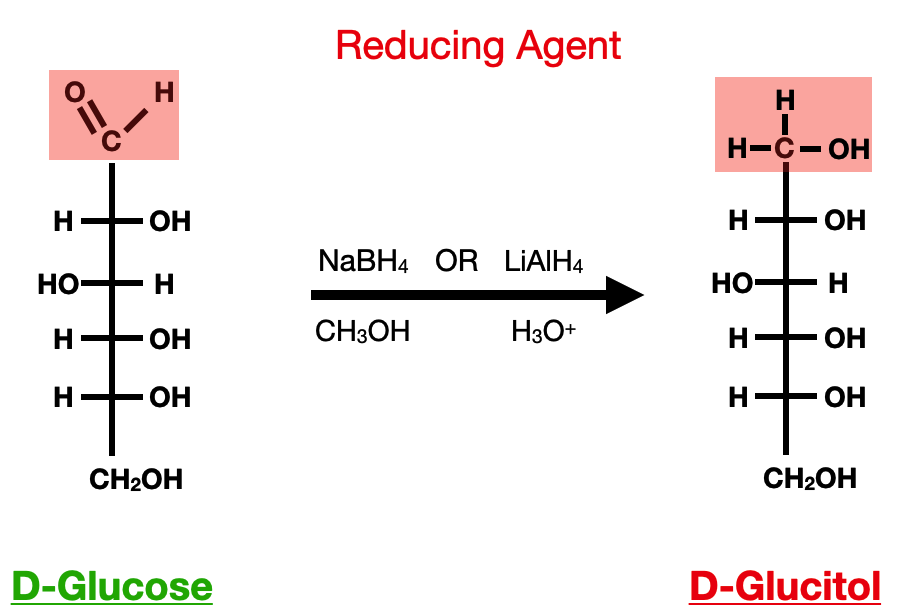
The oxidation and reduction reactions of carbohydrates are closely linked to their role as a source of energy in living organisms. During cellular respiration, the oxidation of glucose generates ATP, which is the main energy currency of cells. The reduction of carbohydrates, such as glucose, also plays a crucial role in biosynthetic pathways, allowing the formation of complex biomolecules such as lipids, proteins, and nucleic acids.
Acetal Formation and Hydrolysis
Acetal formation is an important reaction in the chemistry of saccharides. It involves the conversion of a carbonyl group, typically an aldehyde or ketone, into an acetal functional group. In the context of saccharides, acetal formation occurs when the carbonyl group of a monosaccharide reacts with an alcohol in the presence of an acid catalyst. This reaction leads to the formation of a cyclic acetal linkage, often referred to as a glycosidic bond. The acetal can be hydrolyzed with water/acid to revert back to the hemiacetal form:
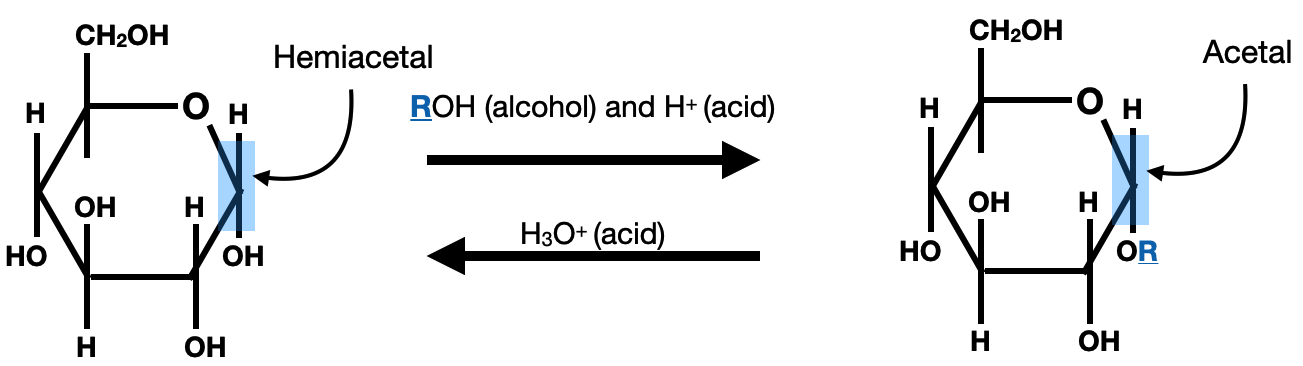
Chain Elongation and Chain Reduction
Chain Elongation
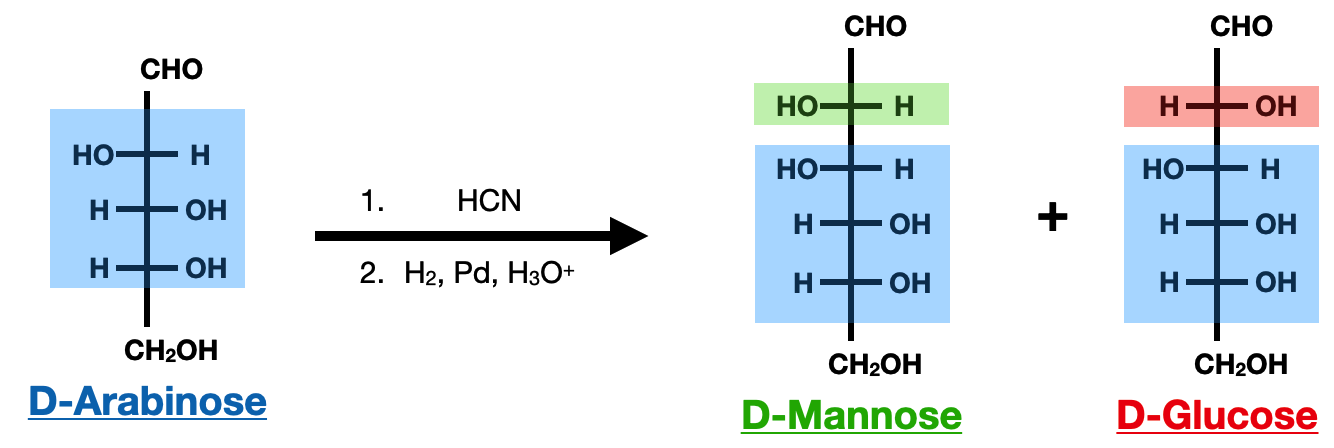
The Kiliani-Fischer synthesis is a prominent method for the preparation of saccharides, specifically aldoses, which are a type of monosaccharide. This synthetic approach allows for the elongation of a carbohydrate chain by one carbon atom. The process involves the conversion of a readily available aldose, such as glyceraldehyde or dihydroxyacetone, into a longer chain aldose.
The Kiliani-Fischer synthesis starts with the reaction of the aldose with cyanide ions to form a cyanohydrin intermediate. This intermediate is then hydrolyzed under acidic conditions to yield a mixture of the corresponding aldonic acid and lactone. The lactone can be further reduced to obtain the desired elongated aldose. One drawback of this method is that it produces a racemic mixture at the end, resulting in two different sugar molecules as this reaction is nonspecific.
Chain Reduction
The Ruff degradation is a chemical method used for the degradation of saccharides, specifically aldoses. It involves the oxidative cleavage of a saccharide chain, resulting in the formation of shorter fragments. This degradation method is particularly useful for the analysis and structural elucidation of complex carbohydrates.
In the Ruff degradation, the aldose is treated with a strong oxidizing agent, such as bromine (Br2) or chlorine (Cl2) in the presence of water (H2O). The oxidizing agent breaks the saccharide chain, resulting in the formation of carboxylic acid. The carboxylic acid then reacts with a Lewis Acid, such as iron(III) acetate (Fe(OAc)3) or iron(III) chloride (FeCl3), which results in the leaving of CO2 and the formation of an aldehyde group from the next closest hydroxyl group, completing the reaction.
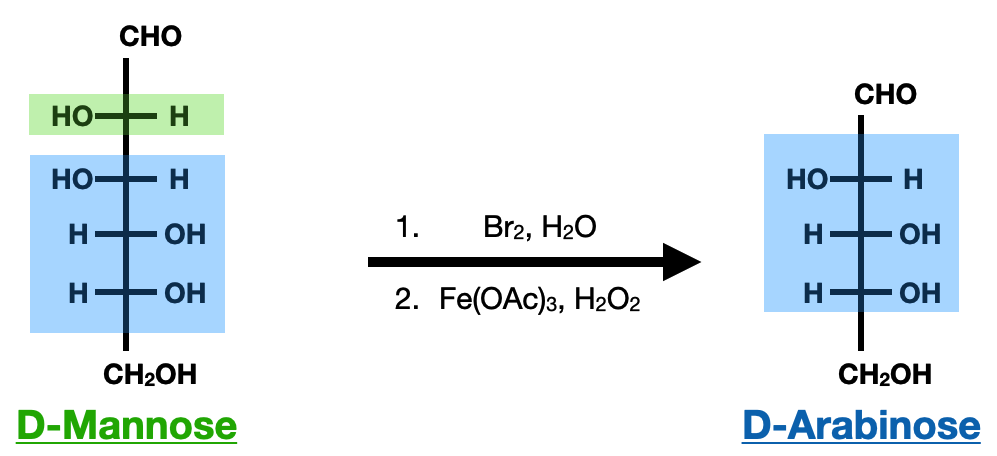
However, it is important to note that the Ruff degradation can introduce side reactions and modifications to the original saccharide structure. Careful control of reaction conditions and subsequent analysis is required to accurately interpret the results and obtain meaningful information about the original saccharide molecule.
Summary
In summation, the study of saccharide reactions is a crucial aspect of understanding the behavior and properties of carbohydrates. This lesson has covered various important reactions and functional groups that are involved in the chemistry of saccharides. We explored oxidation and reduction reactions, which play a key role in modifying the structure and reactivity of saccharides. The Kiliani-Fischer synthesis and Ruff degradation were discussed as powerful methods for the synthesis and degradation of saccharides, respectively.
Additionally, we delved into acetal formation and the significance of protecting groups in saccharide chemistry. These reactions provide a means to selectively modify specific hydroxyl groups, allowing for the synthesis of complex carbohydrate structures.
Understanding these reactions and functional groups is crucial for applications in fields such as medicinal chemistry, carbohydrate synthesis, and glycobiology. By manipulating the structure and reactivity of saccharides, researchers can design novel carbohydrate-based drugs, develop efficient synthetic routes for complex carbohydrates, and study the roles of carbohydrates in biological processes.
Test Your Knowledge
- How can you elongate a saccharide chain? What about shortening it?
- What is the difference between using a mild oxidizing agent and a strong oxidizing agent for monosaccharides? Provide an example of each.