Structure and Nomenclature of Carbohydrates
In the world of carbohydrates, understanding their three-dimensional structures and nomenclature is essential to comprehend their intricate functions. This lesson will delve into Fischer projections, Haworth projections, nomenclature and anomers of saccharides, providing a comprehensive overview of these fundamental concepts. By the end of this lesson, you will gain a deeper understanding of how saccharides are represented, named, and how their structures can vary, particularly in the form of anomers.
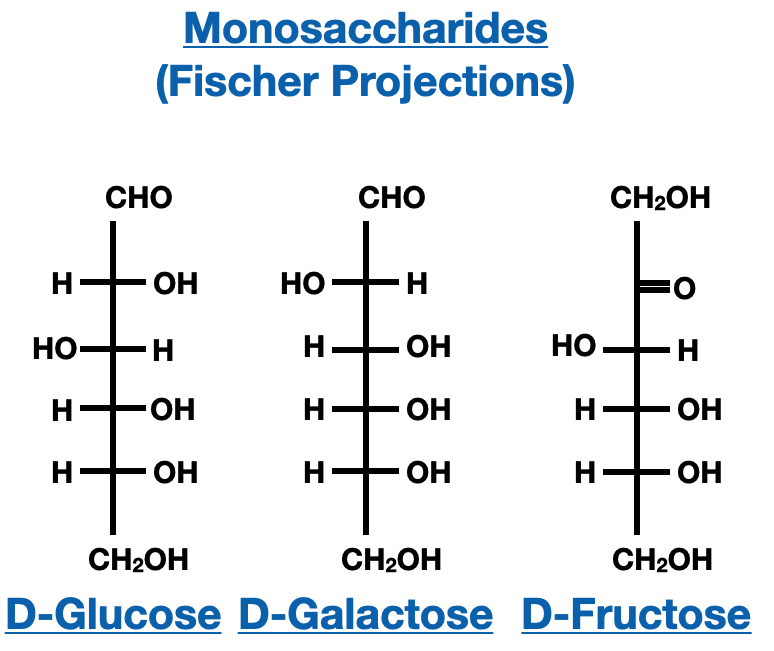
Fischer Projections
Fischer projections are two-dimensional representations of three-dimensional molecules, allowing us to visualize the spatial arrangement of atoms. They are commonly used to depict saccharides and other chiral molecules. Fischer projections provide a simplified view of the molecule, where vertical lines represent bonds projecting away from the viewer, and horizontal lines represent bonds projecting toward the viewer.
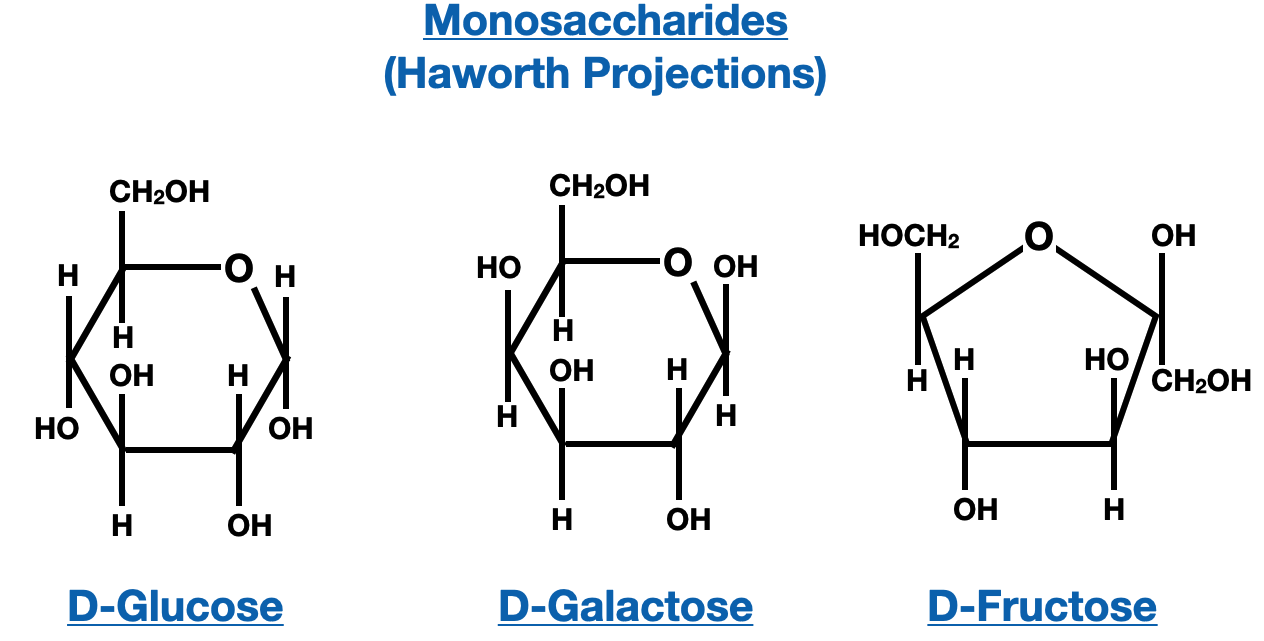
Haworth Projections:
Haworth projections are another important tool for representing saccharides, especially when they exist in cyclic forms. Haworth projections provide a ring representation of saccharides, with the carbon chain arranged in a cyclic structure. In Haworth projections, the carbonyl group is usually depicted at the top of the ring, and hydroxyl groups are either drawn above or below the ring, depending on their orientation.
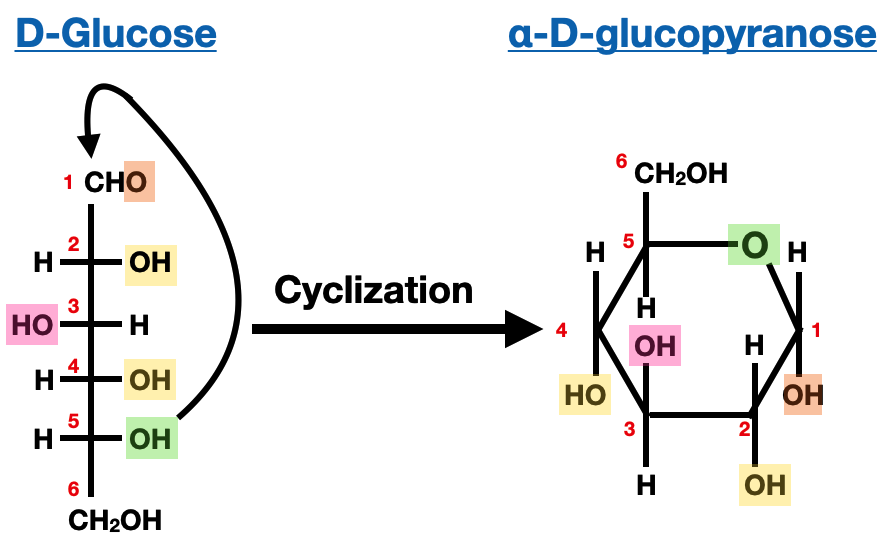
Converting between Fischer and Haworth Projections
Converting between Fischer and Haworth projections is a valuable skill that allows us to translate the two-dimensional representations of saccharides into their cyclic forms and vice versa. To convert from Fischer to Haworth projection, we start by visualizing the carbon chain in the Fischer projection as a ring. The horizontal lines in the Fischer projection represent bonds projecting toward the viewer, while the vertical lines represent bonds projecting away from the viewer. By bending the chain into a cyclic structure, we can create the Haworth projection. The carbon at the top of the Fischer projection becomes the anomeric carbon in the Haworth projection, and the hydroxyl groups are placed either above or below the ring, depending on their orientation.
To convert from Haworth to Fischer projection, we reverse the process. We start with the cyclic structure of the Haworth projection and mentally flatten it out to form a straight carbon chain. The hydroxyl groups that were above or below the ring in the Haworth projection are represented as vertical or horizontal lines in the Fischer projection, respectively. The anomeric carbon in the Haworth projection becomes the carbon at the top of the Fischer projection. By following these guidelines, we can effectively convert between Fischer and Haworth projections, enabling us to visualize and communicate the structures of saccharides in different forms.
Nomenclature of Saccharides:
Monosaccharides, the simplest form of saccharides, are classified based on the number of carbon atoms they contain. The most common monosaccharides, such as glucose, fructose, and galactose, are hexoses, meaning they consist of six carbon atoms. Other monosaccharides can have three carbon atoms (trioses), four carbon atoms (tetroses), five carbon atoms (pentoses), and so on. The naming of monosaccharides also takes into account the stereochemistry of their chiral carbon atoms, which can exist in either a D (dextrorotatory) or L (levorotatory) configuration.
In a cyclic monosaccharide, the carbonyl group (either an aldehyde or a ketone) reacts with a hydroxyl group on another carbon atom within the same molecule, forming a cyclic hemiacetal or hemiketal. The resulting ring can be either a five-membered ring called a furanose or a six-membered ring called a pyranose.
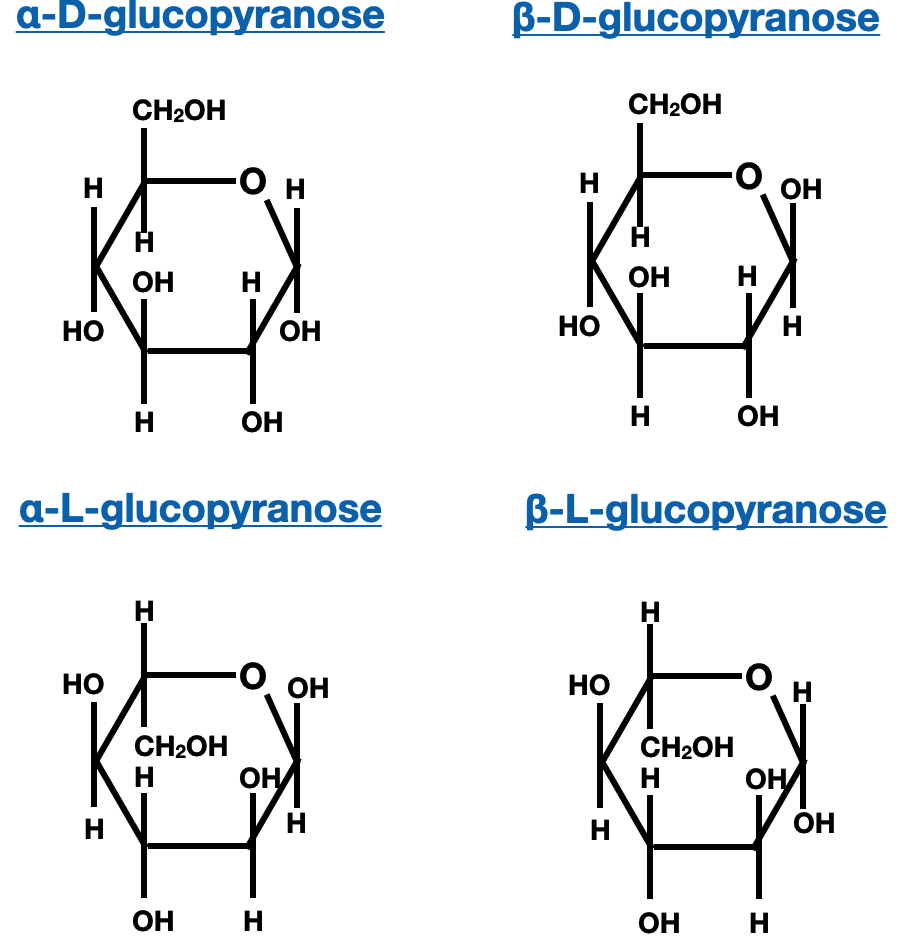
The cyclic form of a monosaccharide is named based on the position of the carbon atom that becomes the anomeric carbon, which is the carbon involved in the formation of the hemiacetal or hemiketal linkage. The anomeric carbon is assigned a specific designation, either α or β, to indicate its stereochemistry. The α-anomer refers to the configuration where the hydroxyl group on the anomeric carbon is pointing in the opposite direction (downward) from the ring plane, while the β-anomer indicates the hydroxyl group is pointing in the same direction (upward) as the ring plane.
The names of cyclic monosaccharides are derived by combining the prefix indicating the number of carbon atoms (e.g., "pent" for five carbons) with the suffix "ose" to denote a sugar. For example, a cyclic monosaccharide with six carbon atoms in the ring and an aldehyde group is called an aldohexose. The specific name of the monosaccharide, such as glucose or fructose, is added as a prefix to indicate the particular structure, such as glucopyranose or fructofuranose.
Anomers of Saccharides:
Anomers are a specific type of stereoisomers that differ in the configuration of the anomeric carbon, which is the carbon that becomes a stereocenter upon ring closure of a saccharide. Anomers can exist in two forms: alpha (α) and beta (β), depending on the orientation of the anomeric hydroxyl group. Anomers play a crucial role in the properties and reactivity of saccharides.
Summary
In this lesson, we explored the nomenclature of saccharides, focusing on monosaccharides and their cyclized forms. We learned that when a monosaccharide undergoes cyclization, it forms a ring structure known as a furanose or a pyranose. The cyclic form of a monosaccharide is named based on the position of the anomeric carbon and the stereochemistry of the hydroxyl group on that carbon. The nomenclature also involves numbering the carbon atoms in the ring and using specific prefixes and suffixes to indicate the number of carbons and the type of sugar. Understanding the nomenclature of cyclic monosaccharides is essential for accurately describing their structures and identifying different sugars.
Test Your Knowledge:
What is the difference between a furanose and a pyranose?
How is the stereochemistry of the anomeric carbon indicated in the nomenclature of cyclic monosaccharides?