Amino Acid Synthesis and Protection Reactions
Amino acids are essential building blocks of proteins and play vital roles in various biochemical processes. Organic chemists have developed several methods to synthesize amino acids in the laboratory. One method involves using the Hell-Volhard-Zelinsky (HVZ) reaction in combination with the Gabriel synthesis, which involves the transformation of alpha-halo acids or esters into amino acids through a multi-step reaction sequence. Another method is known as the Strecker synthesis which takes an aldehyde and converts it to an amino acid using ammonia, followed by a cyanide ion, and ends with an acid hydrolysis. Additionally, during the synthesis process, it is crucial to protect the reactive functional groups, such as the amino and carboxylic acid groups, to prevent undesired side reactions and ensure the desired product formation.
Hell-Volhard-Zelinsky (HVZ) reaction and Gabriel synthesis
Hell-Volhard-Zelinsky Reaction:
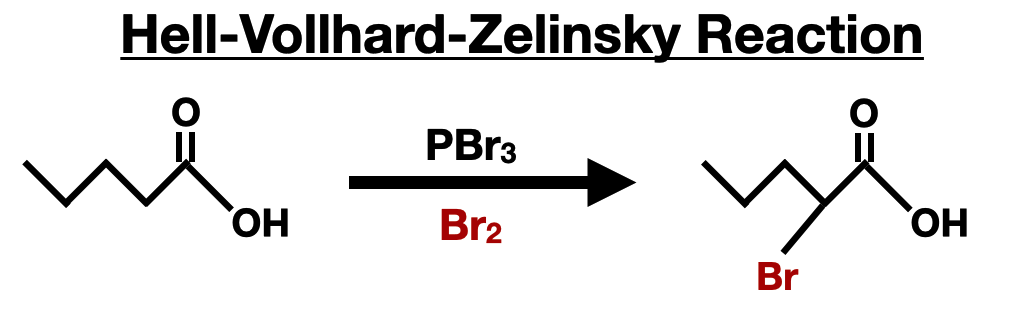
The Hell-Volhard-Zelinsky (HVZ) reaction is a versatile method for the introduction of a carboxylic acid group into an alpha-carbon of a compound containing a hydrogen atom in the alpha position. This reaction is particularly useful for the synthesis of alpha-halo acids, which serve as key intermediates for amino acid synthesis.
In the HVZ reaction, the alpha-hydrogen of a carboxylic acid or an acid derivative is replaced by a halogen atom (chlorine or bromine) using a halogenating agent, such as phosphorus trichloride (PCl3) or phosphorus tribromide (PBr3), and the respective halogen such as chlorine (Cl2) or bromine (Br2). The reaction proceeds through the formation of an acyl halide intermediate, which subsequently undergoes halogenation at the alpha-carbon position.
Gabriel Synthesis:
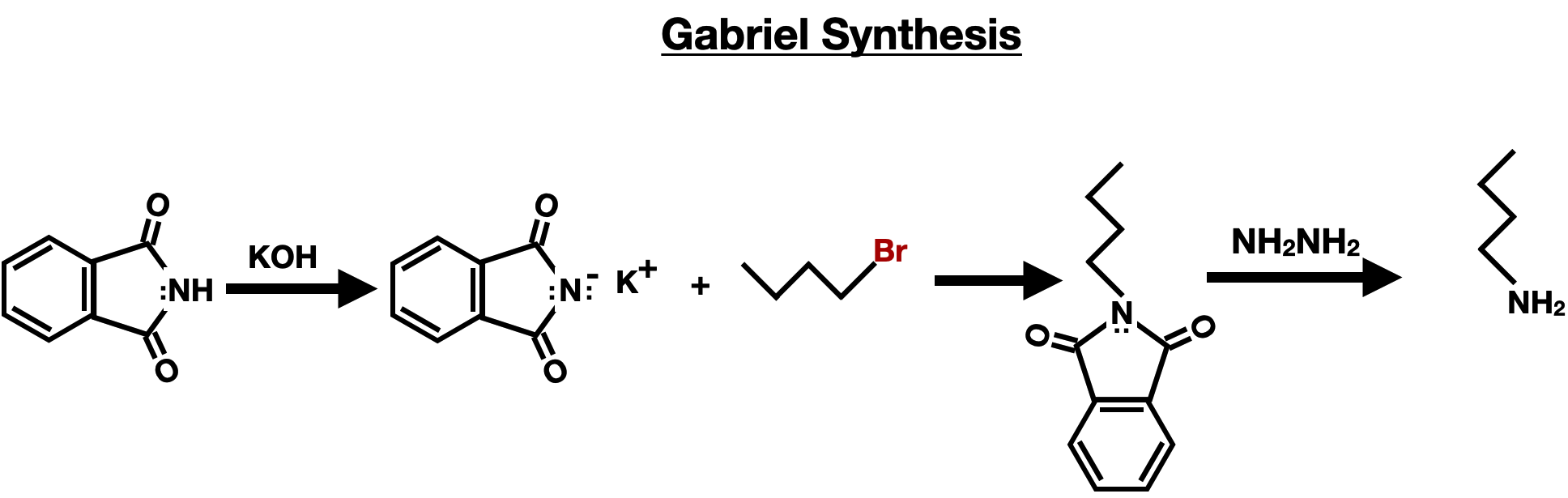
The Gabriel Synthesis is a method for the preparation of primary amines, including amino acids. It involves the reaction of a phthalimide derivative with an alkyl halide in the presence of a strong base, such as potassium hydroxide (KOH). The reaction forms an N-alkyl phthalimide intermediate, which is then hydrolyzed with a strong base, such as hydrazine (NH2NH2), to release the desired primary amine.
In the context of amino acid synthesis, the N-alkyl phthalimide intermediate acts as a protected form of the amino acid, where the amine group is masked. The final step of the Gabriel synthesis involves the hydrolysis of the N-alkyl phthalimide, revealing the free amine group and yielding the corresponding amino acid.
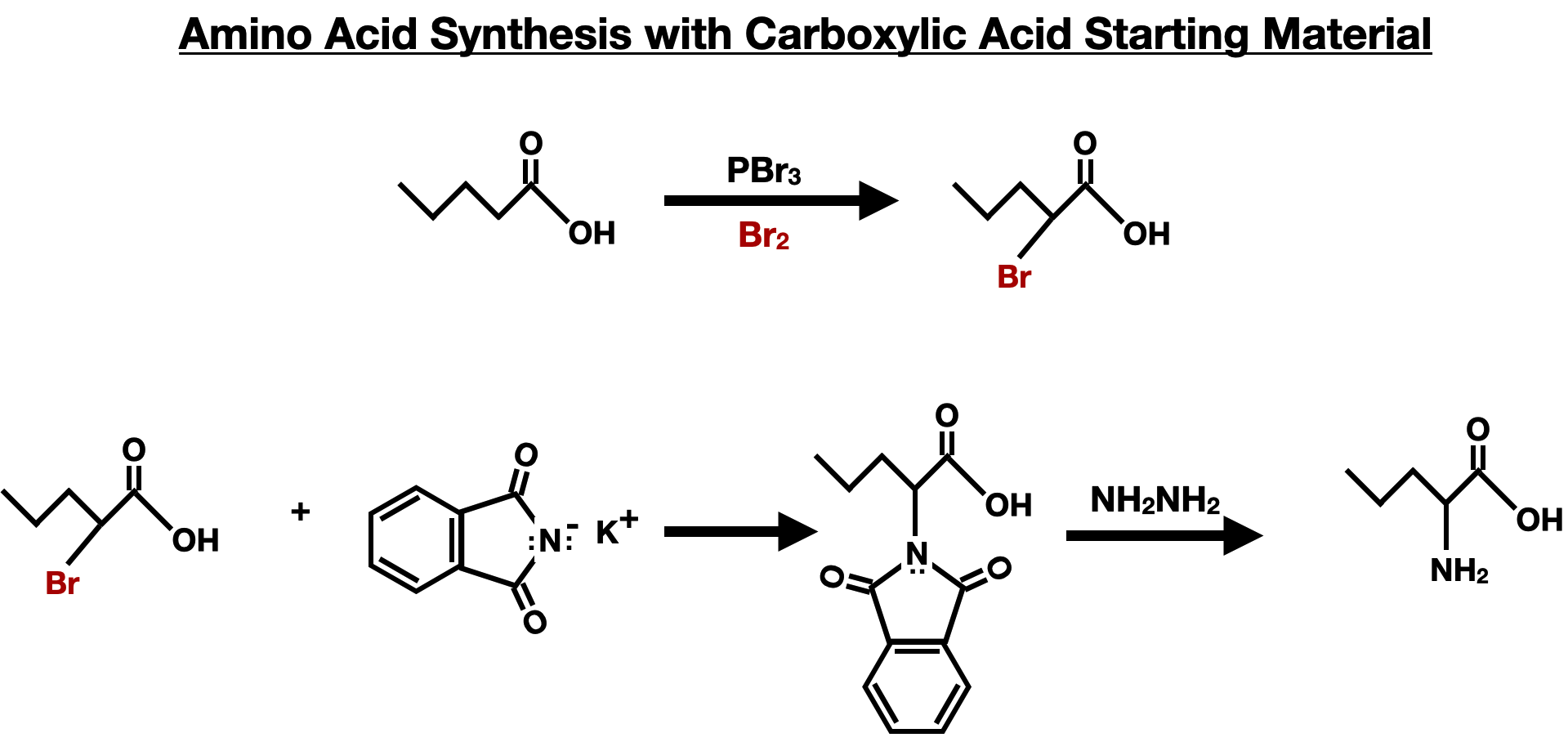
HVZ and Gabriel Combined
Strecker Synthesis
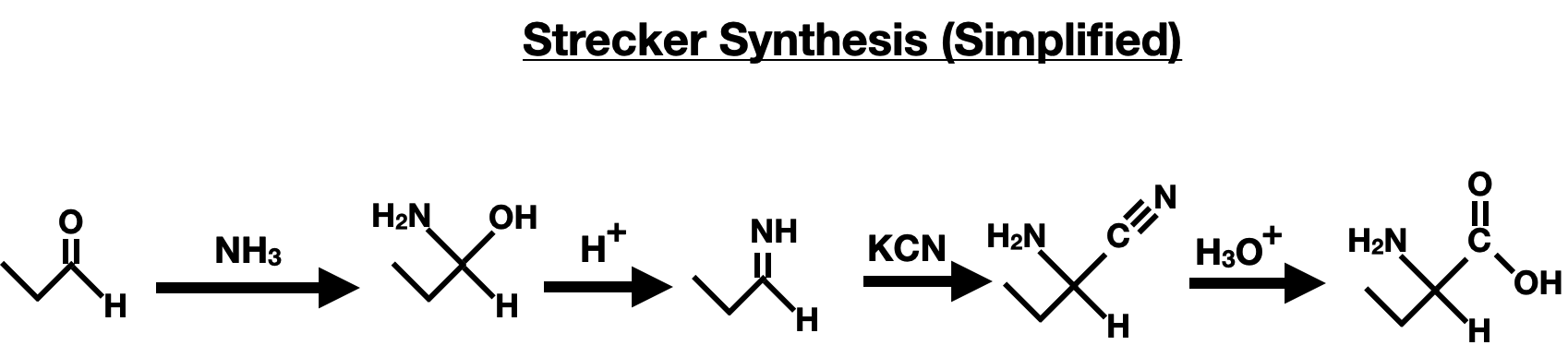
The Strecker Synthesis is a classical method used to synthesize alpha-amino acids from aldehydes or ketones. It involves the reaction of an aldehyde or ketone with ammonia (NH3) and potassium cyanide (KCN). The reaction proceeds through a multistep process, resulting in the formation of an alpha-amino nitrile intermediate, which can then be hydrolyzed to yield the corresponding alpha-amino acid.
The Strecker Synthesis starts with the nucleophilic attack from ammonia on the carbonyl carbon of the aldehyde or ketone, forming a bifunctional molecule that has a hydroxyl (-OH) and an amine (-NH2) group. The next step involves protonation of the hydroxly group by an acid (H+), resulting in the elimination of water, leading to the formation of the iminium ion intermediate. A cyanide ion (CN-) attacks the iminium carbon, forming an aminonitrile. The aminonitrile ungerdoes acidic hydrolysis, yielding the final product of an amino acid.
Protecting Reactions
During the amino acid synthesis, it is crucial to protect the amino and carboxylic acid groups to avoid unwanted side reactions. The amino group can be protected using various protecting groups like benzyl or tert-butoxycarbonyl (Boc), while the carboxylic acid group can be protected using esterification to form an alkyl ester.
Amino Group Protection
To protect the amino group, the alpha-amino acid is treated with an appropriate protecting reagent, such as benzyl chloride or Boc anhydride, resulting in the formation of a benzyl or Boc-protected amino acid.
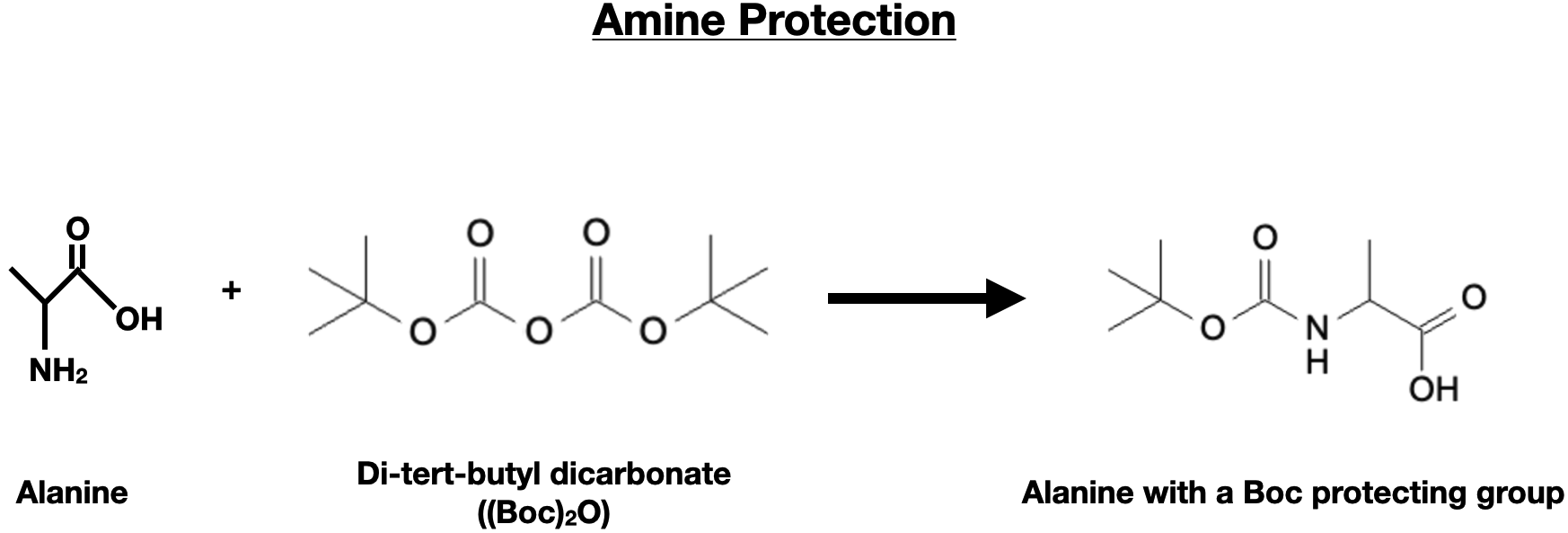
Carboxylic Acid Group Protection
The carboxylic acid group is protected by converting it into an alkyl ester through esterification with an alcohol and an acid catalyst.
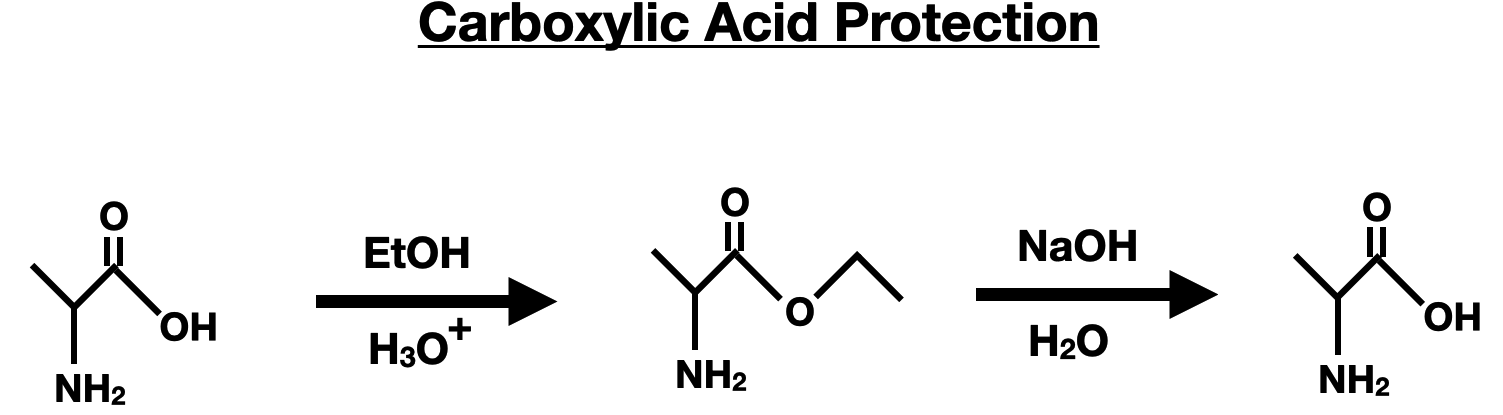
Summary
The Hell-Volhard-Zelinsky/Gabriel synthesis and Strecker synthesis are valuable methods for the efficient synthesis of amino acids from alpha-halo acids, esters, aldehydes, and ketones. Protecting reactions are essential during the synthesis process to preserve the reactivity of functional groups and ensure the formation of the desired product. By carefully designing and executing these reactions, organic chemists can access a wide range of amino acids, contributing to advancements in various fields, including medicine, biochemistry, and pharmaceuticals.
Test Your Knowledge
- Why is protecting amino acids helpful?
- What is the Strecker synthesis and how does it proceed?