The Twenty Amino Acids
Amino acids are the fundamental building blocks of proteins, which are essential for the structure and function of all living organisms. These small organic molecules play a crucial role in various biological processes, ranging from enzyme catalysis to cell signaling. Understanding the properties and characteristics of amino acids is fundamental to comprehend protein structure, function, and the intricate mechanisms that govern cellular activities. In this lesson, we will delve into the world of amino acids, specifically focusing on the twenty amino acids that are commonly found in proteins. We will explore the different groups of amino acids, their unique properties, and their significance in protein structure and function.
The Twenty Amino Acids:
The 20 amino acids are the building blocks of proteins and play a critical role in biological processes. Each amino acid is characterized by its unique side chain, also known as the R-group, which differentiates one amino acid from another. The side chain determines the chemical and physical properties of the amino acid, contributing to its overall function and behavior.
The 20 amino acids can be broadly categorized into several groups based on the nature of their side chains. These categories include:
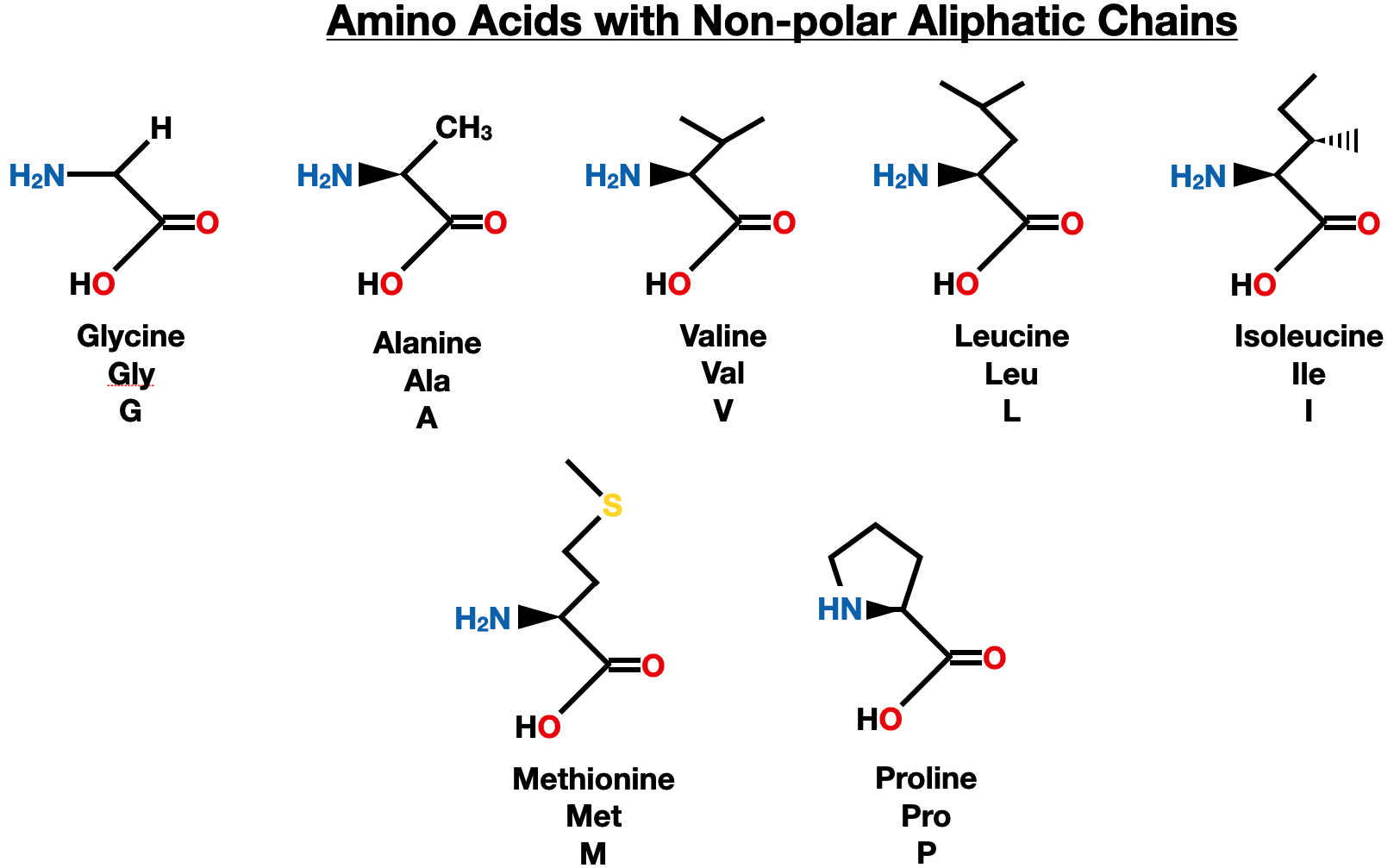
Non-polar Aliphatic Side Chains
Non-polar, aliphatic side chains refer to a group of amino acids that have hydrophobic properties and are primarily composed of carbon and hydrogen atoms. Aliphatic refers to a class of organic compounds characterized by straight or branched carbon chains. These side chains lack any charged or polar groups, which makes them less likely to interact with water molecules. Instead, they tend to be buried within the protein structure, shielded from the aqueous environment.
Glycine, the simplest amino acid, has a hydrogen atom as its side chain. It is highly flexible due to the absence of bulky side chains and can be found in tight turns or bends in protein structures.
Alanine is a small amino acid with a methyl group as its side chain. It is commonly found in the interior of proteins, contributing to their stability by packing tightly with other non-polar residues.
Valine, Leucine, and Isoleucine are branched-chain amino acids with longer side chains. They are hydrophobic in nature and are often involved in protein-protein interactions and the formation of hydrophobic cores within proteins.
Methionine is an essential amino acid with a non-polar, aliphatic side chain. It is known as the initiator amino acid as it often serves as the starting point for protein synthesis.
Proline is a unique amino acid with a non-polar, aliphatic side chain that forms a cyclic structure. It is distinguished by its imino group, which forms a ring with the amino group, resulting in a secondary amine. Proline has a rigid structure that disrupts the typical peptide bond configuration, making it an important determinant of protein folding and stability. It is commonly found in the structural regions of proteins, such as collagen, where its presence helps maintain the structural integrity of tissues.
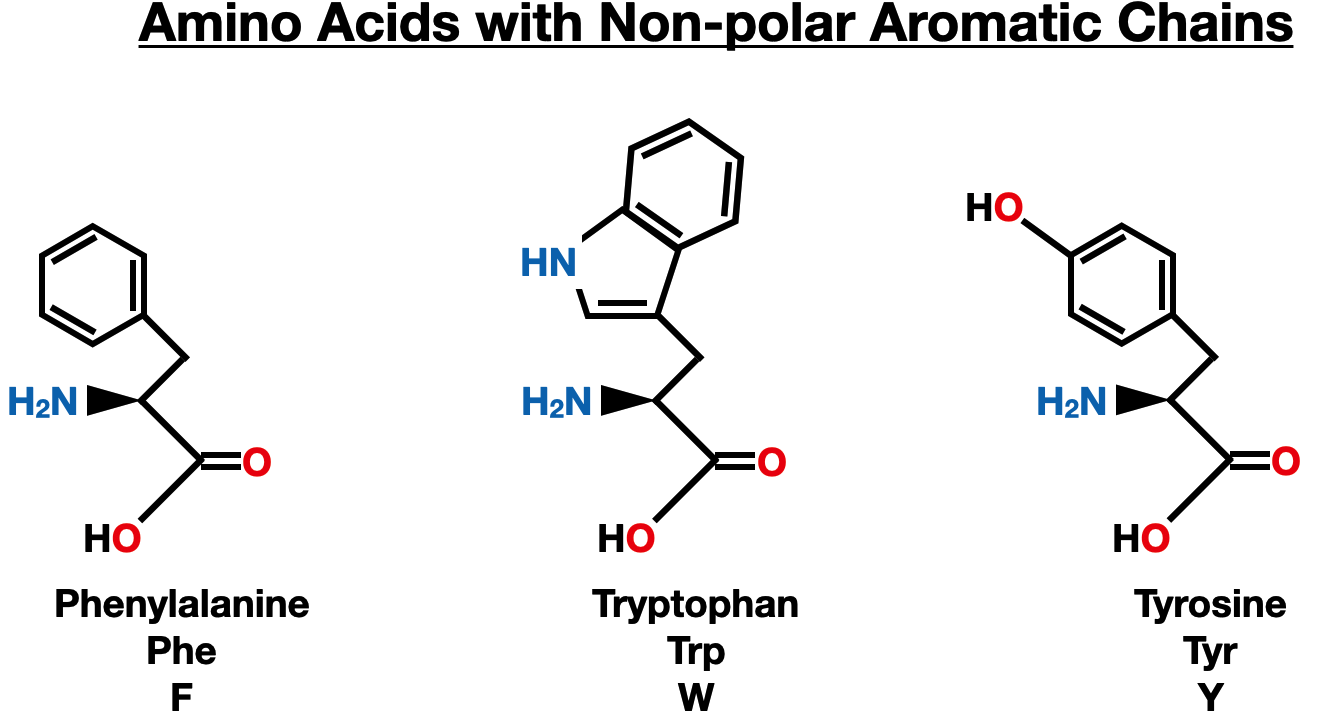
Non-polar Aromatic Side Chains
Phenylalanine (Phe), tryptophan (Trp), and tyrosine (Tyr) are three amino acids with non-polar aromatic side chains. These aromatic side chains contribute to their unique chemical and biological properties.
Phenylalanine (Phe) is an essential amino acid that plays a crucial role in protein synthesis. It has a benzyl side chain, which consists of a phenyl group. Phenylalanine is involved in various biochemical processes and serves as a precursor for the synthesis of neurotransmitters such as dopamine and norepinephrine.
Tryptophan (Trp) is an essential amino acid and is the largest amino acid in terms of molecular weight. It contains an indole ring in its side chain, which gives it unique chemical properties. Tryptophan is a precursor for the synthesis of serotonin and melatonin, which are important neurotransmitters and regulators of sleep and mood.
Tyrosine (Tyr) is a nonessential amino acid that contains a phenol group in its side chain. It is synthesized from phenylalanine in the body. Tyrosine is involved in the production of important compounds such as dopamine, adrenaline, and thyroxine. It also plays a role in protein phosphorylation, which regulates cellular processes.
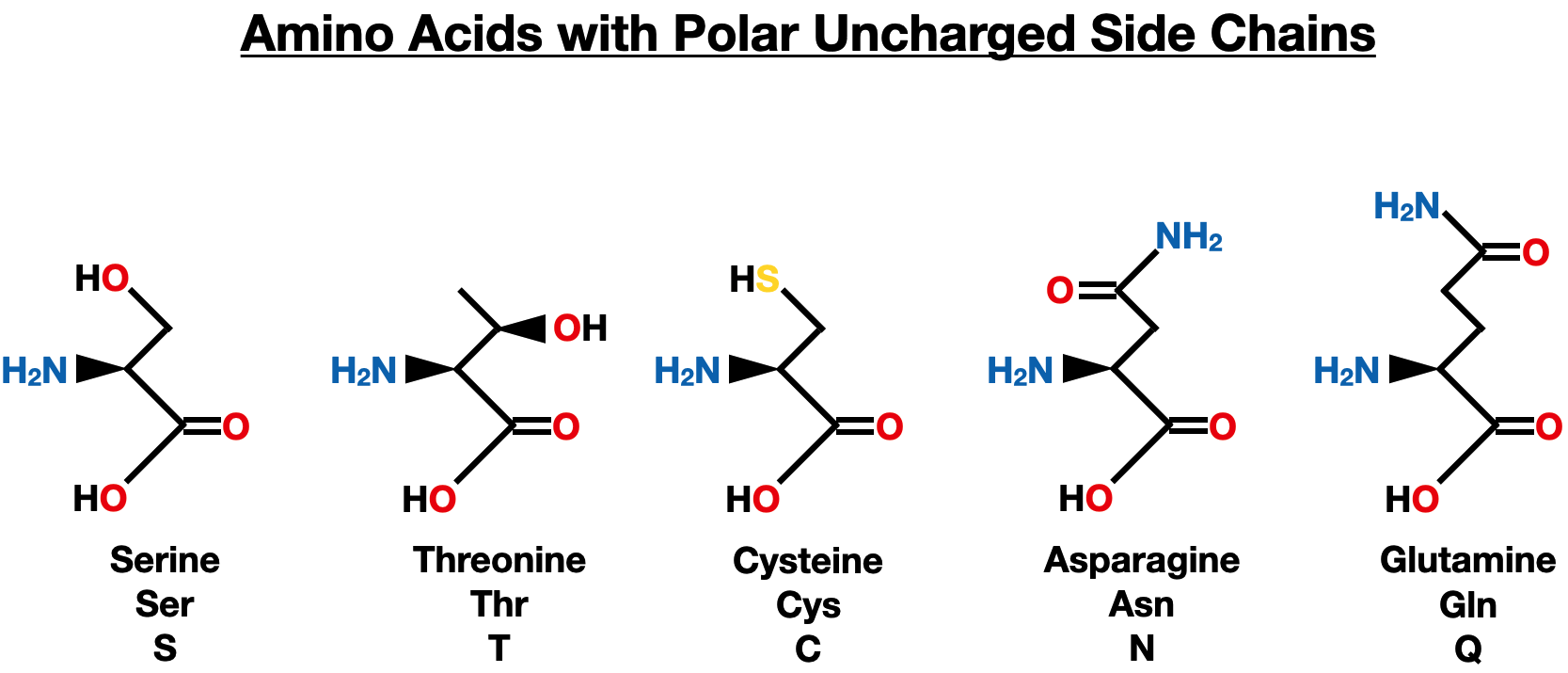
Polar Uncharged Side Chains
Serine, threonine, cysteine, asparagine, and glutamine are five amino acids with polar, uncharged side chains. These side chains have unique chemical properties that play crucial roles in the structure and function of proteins.
Serine (Ser) and threonine (Thr) are both polar amino acids with hydroxyl groups (-OH) in their side chains. They are important for protein synthesis and have significant roles in enzymatic reactions. Serine is involved in the catalytic activity of various enzymes, while threonine is essential for the formation of collagen and elastin, which are structural proteins.
Cysteine (Cys) is a polar amino acid that contains a thiol group (-SH) in its side chain. This thiol group enables cysteine to form disulfide bonds with another cysteine residue, contributing to the stability of protein structures and protein folding. Cysteine is essential for maintaining protein structure and is found in various antioxidant enzymes.
Asparagine (Asn) and glutamine (Gln) have polar side chains containing amide groups. They are crucial for protein synthesis and are involved in various cellular processes. Asparagine plays a role in glycosylation, which modifies proteins for specific functions, while glutamine is important for nitrogen transport and metabolism.
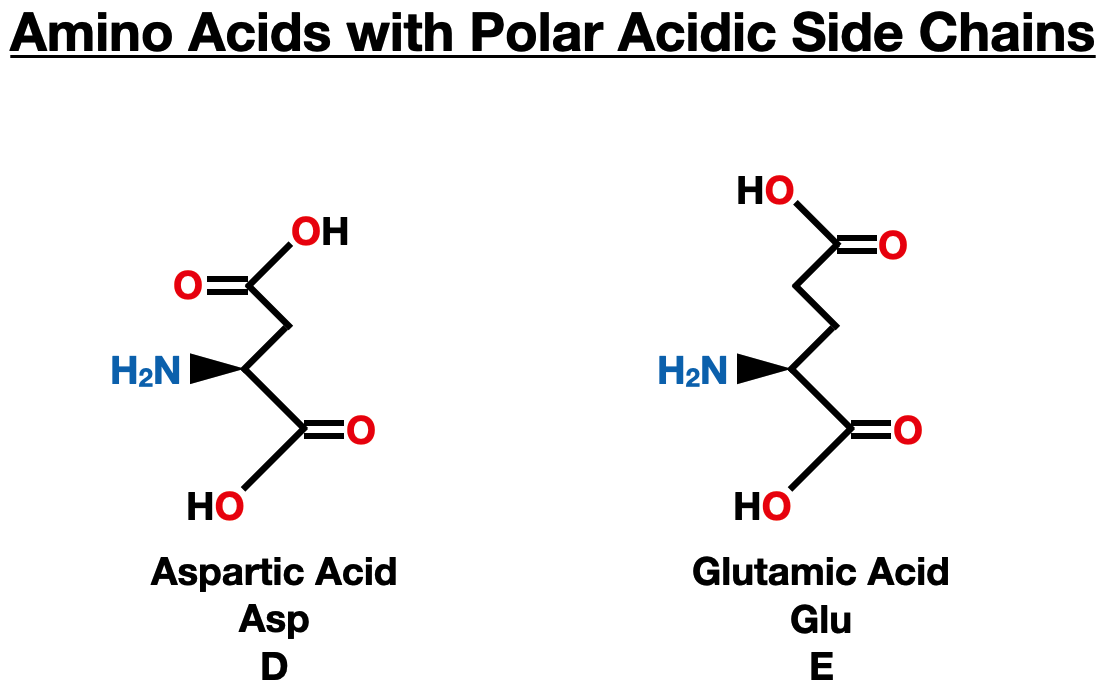
Polar Acidic Side Chains
Aspartic acid (Asp) and glutamic acid (Glu) are two amino acids with polar, acidic side chains. Their side chains contain carboxyl groups (-COOH), which are responsible for their acidic nature. These amino acids play crucial roles in protein structure, function, and cellular signaling.
Aspartic acid is involved in many enzymatic reactions and serves as a crucial component of active sites in enzymes. It plays a vital role in the catalytic activity of various proteins, contributing to the acceleration of chemical reactions. Aspartic acid also participates in neurotransmission as an excitatory neurotransmitter in the central nervous system, influencing synaptic signaling.
Glutamic acid is abundant in proteins, and its acidic side chain contributes to the negative charge on the protein surface. This negative charge can attract positively charged ions or molecules, influencing protein-protein interactions and protein folding. Glutamic acid is also an important neurotransmitter in the brain, acting as the primary excitatory neurotransmitter in the central nervous system and playing a significant role in memory and learning.
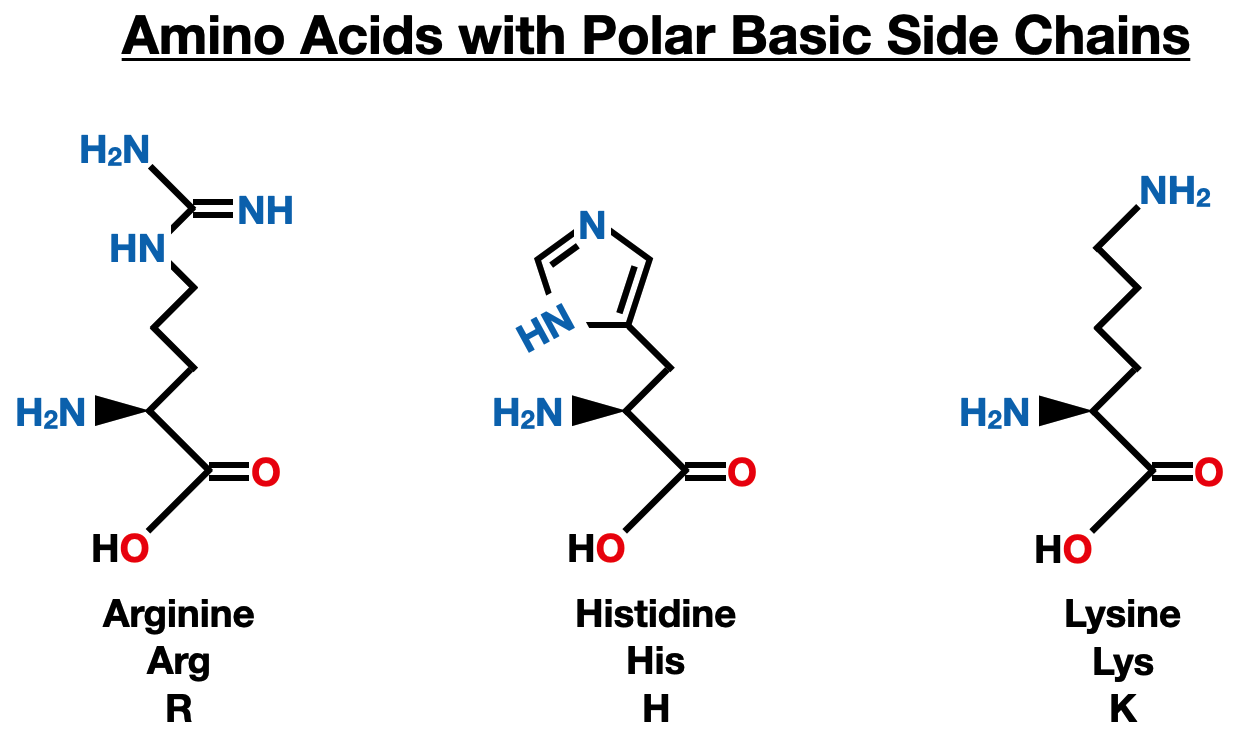
Polar Basic Side Chains
Arginine (Arg), histidine (His), and lysine (Lys) are amino acids with polar, basic side chains. These side chains contain amino groups (-NH2) or guanidinium groups, which impart a positive charge to the side chain at physiological pH. The basic nature of these amino acids makes them important for various biological processes.
Arginine is involved in several crucial functions, including protein synthesis, hormone secretion, and wound healing. It serves as a precursor for the production of nitric oxide (NO), a potent vasodilator that regulates blood vessel dilation and blood pressure. Arginine is also a key component of histones, the proteins that help package and organize DNA within the cell nucleus.
Histidine plays a critical role in enzyme catalysis, as it can serve as a proton donor or acceptor in enzymatic reactions. Its imidazole side chain allows it to act as a pH buffer, stabilizing the active sites of certain enzymes under acidic or basic conditions. Histidine is also involved in the binding and transport of metal ions, playing a role in heme-containing proteins like hemoglobin and myoglobin.
Lysine is essential for protein synthesis, as it is involved in post-translational modifications like acetylation and methylation. It is crucial for the proper structure and function of collagen, the most abundant protein in the human body, and is involved in the formation of cross-links in collagen fibers, contributing to the strength and stability of connective tissues.
Why are all amino acids in the L configuration?
The predominance of amino acids in the L configuration, as opposed to the D configuration, in biological systems can be attributed to the historical development of life on Earth. This phenomenon is often referred to as the "chirality of life."
The stereochemistry of amino acids is based on their asymmetric carbon atom, called the alpha carbon, which is bonded to four different groups: the amino group (-NH2), the carboxyl group (-COOH), a hydrogen atom (-H), and the side chain specific to each amino acid. The arrangement of these groups determines the chiral nature of the molecule.
In the early stages of Earth's history, before the emergence of life, it is believed that both L and D amino acids were present in equal amounts. However, when life began to evolve, a selective preference for the L configuration occurred. This selectivity might have been influenced by environmental factors or the specific biochemical processes involved in the origin and development of life.
Once the preference for L amino acids was established in early life forms, it became perpetuated through the evolutionary processes. Enzymes, which are responsible for the synthesis and metabolism of amino acids, exhibit specificity for L amino acids. Over time, this selective pressure led to the predominance of L amino acids in biological systems.
The specific reasons why L amino acids were favored over D amino acids in the evolution of life are still a subject of scientific investigation. It is likely a combination of factors including the selective binding to enzymes and receptors, compatibility with other biomolecules, and stability of resulting protein structures.
It's important to note that D amino acids do exist in certain biological systems, such as some bacterial cell walls and certain peptides found in marine organisms. However, the vast majority of proteins and the amino acids they are composed of exhibit the L configuration in living organisms.
Summary
The 20 amino acids discussed above play crucial roles in the structure, function, and regulation of proteins, making them fundamental building blocks of life. Their diverse side chains confer a wide range of chemical properties and functional groups, allowing them to participate in various biochemical reactions. These amino acids are essential for protein synthesis, enzyme catalysis, cell signaling, and maintaining the structural integrity of tissues.
Overall, the knowledge of amino acids and their organic chemistry implications is crucial for comprehending the molecular basis of life and for advancing fields such as biochemistry, molecular biology, pharmacology, and medicine. The intricate interplay of organic chemistry principles with biological systems continues to drive groundbreaking research and innovations in the quest to understand and improve human health and the living world as a whole.
Test your knowledge
- What are the 5 categories of amino acids?
- What configuration are all of the amino acids in? Why?