Dienes: Understanding Stability, Resonance Energy, and Kinetic vs Thermodynamic Control
Dienes, a subclass of alkenes, are hydrocarbons with two carbon-carbon double bonds. They play a crucial role in organic chemistry due to their unique reactivity and properties. In this lesson, we will explore the factors that influence the stability of different dienes, delve into the concept of resonance energy, and discuss the intriguing aspects of kinetic and thermodynamic control in diene reactions.
Stability of Different Dienes
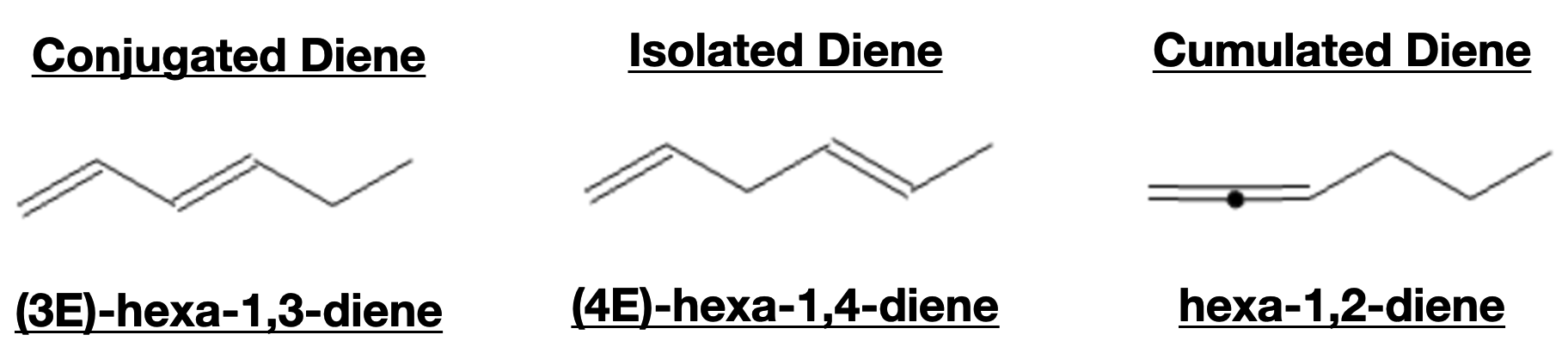
The stability of a diene largely depends on its molecular structure and the spatial arrangement of the double bonds. Dienes can be classified into three main categories: isolated dienes, conjugated dienes, and cumulated dienes.
Isolated Dienes:
Isolated dienes have no additional double bonds nearby. Due to the absence of conjugation, they are less stable compared to conjugated dienes. The isolated dienes have increased electron density on the double bonds, making them more reactive towards electrophilic additions.
Conjugated Dienes:
Conjugated dienes have alternating single and double bonds, leading to the sharing of pi electrons across all double bonds. This conjugation imparts higher stability to the molecule, reducing the electron density on individual double bonds. Conjugated dienes exhibit resonance, which results in delocalization of electrons, lowering the overall energy of the system.
Cumulated Dienes:
Cumulated dienes have two double bonds adjacent to each other, sharing a common carbon. These dienes have the least stability among the three categories due to strong repulsive interactions between the adjacent double bonds. As a result, cumulated dienes are less commonly encountered in organic chemistry reactions.
Resonance Energy of Dienes
The resonance energy of dienes refers to the stabilization energy achieved through electron delocalization in conjugated systems. Conjugated dienes have a higher resonance energy compared to isolated dienes, making them more stable. This increased stability is attributed to the delocalization of pi electrons across the entire conjugated system. The greater the number of conjugated double bonds, the higher the resonance energy and the overall stability of the molecule.
The presence of resonance in conjugated dienes lowers the overall energy of the system, making them more stable compared to isolated or cumulated dienes. The stabilization energy gained from resonance is referred to as resonance energy. It is a measure of the difference in energy between the actual molecule (which exists in multiple resonance forms) and the most stable hypothetical structure based on localized double bonds.
The greater the number of conjugated double bonds in a diene, the higher the resonance energy, leading to increased stability. This is because a larger conjugated system allows for more extensive delocalization of pi electrons, distributing the electron density more evenly along the molecule. Consequently, conjugated dienes are more resistant to reactions that would disrupt the conjugation, making them important intermediates in various organic reactions.
Kinetic and Thermodynamic Control of Dienes
In chemical reactions involving dienes, two different modes of product formation are observed based on the reaction conditions: kinetic control and thermodynamic control. These concepts are essential in understanding the selectivity and outcome of diene reactions.
Kinetic Control:
Kinetic control refers to the formation of products that are favored by the reaction conditions, particularly at lower temperatures or shorter reaction times. In kinetic control, the reaction occurs rapidly, and the product is formed quickly without sufficient time for the system to reach its thermodynamic equilibrium. As a result, the product formed is usually the one that has the lowest activation energy, which may not necessarily be the most stable product.
In diene reactions under kinetic control, the reaction typically proceeds through a pathway that leads to the formation of a less substituted or more reactive intermediate. This intermediate is formed more rapidly due to its lower activation energy. As a consequence, the final product is often the one derived from this less stable intermediate, even though a more stable product might be possible at thermodynamic equilibrium.
Thermodynamic Control:
Thermodynamic control, on the other hand, occurs when the reaction conditions allow sufficient time for the system to reach its thermodynamic equilibrium. This typically involves higher temperatures or longer reaction times. Under thermodynamic control, the product formed is the one that is most stable and has the lowest free energy (highest thermodynamic stability) among the possible products.
In diene reactions under thermodynamic control, the reaction proceeds through a pathway that leads to the formation of a more substituted or more stable intermediate, even though this intermediate might have a higher activation energy. As the reaction progresses to thermodynamic equilibrium, the less stable intermediate is converted to the more stable product.
The kinetic and thermodynamic control of diene reactions can lead to different products with distinct stereochemical outcomes. Kinetic products are often formed rapidly and can be kinetically favored due to the presence of a fast-reacting intermediate. Thermodynamic products, on the other hand, are formed over a longer time frame, and their stability is dictated by the relative stability of the products at thermodynamic equilibrium.
Summary
In summary, dienes are remarkable entities that offer a fascinating array of stability and reactivity traits. The knowledge of resonance energy and the concepts of kinetic and thermodynamic control empowers chemists to unlock the full potential of dienes in organic synthesis. By utilizing these principles, researchers can craft efficient and selective routes to various valuable chemical products, driving innovation and progress in the field of organic chemistry. As our understanding of dienes continues to evolve, so does the potential for the discovery of novel chemical reactions and the development of innovative applications across diverse scientific disciplines.
Test Your Knowledge:
Differentiate between kinetic control and thermodynamic control in diene reactions. How does the reaction progress under each mode, and what factors influence the formation of kinetic and thermodynamic products in diene reactions?
How does resonance energy contribute to the stability of conjugated dienes? Explain the relationship between the number of conjugated double bonds and the resonance energy of a diene.