Introduction to Dienes
Dienes represent a fascinating class of organic compounds that play a crucial role in organic chemistry. These molecules are characterized by having two double bonds, which provide unique reactivity and stereochemical properties. In this lesson, we will delve into the world of dienes, exploring their structure, nomenclature, and significance in various chemical reactions.
Structure and Nomenclature of Dienes
Dienes are hydrocarbons with two consecutive double bonds, which can be present in either a conjugated or non-conjugated arrangement. In the conjugated dienes, the two double bonds are separated by a single carbon-carbon bond, leading to a continuous system of overlapping p orbitals. This results in increased stability and unique reactivity compared to non-conjugated dienes. On the other hand, non-conjugated dienes have at least 2 carbon-carbon single bonds between the two double bonds, leading to separate systems of pi bonds.
Nomenclature of dienes follows the rules of alkene nomenclature, with the presence of two double bonds indicated by the suffix "-adiene." For example, a conjugated diene with four carbon atoms would be named as buta-1,3-diene. Non-conjugated dienes are named similarly but with a number indicating the positions of the double bonds. For instance, a non-conjugated diene with five carbon atoms and double bonds at positions 1 and 4 would be named as penta-1,4-diene.

Reactivity of Dienes
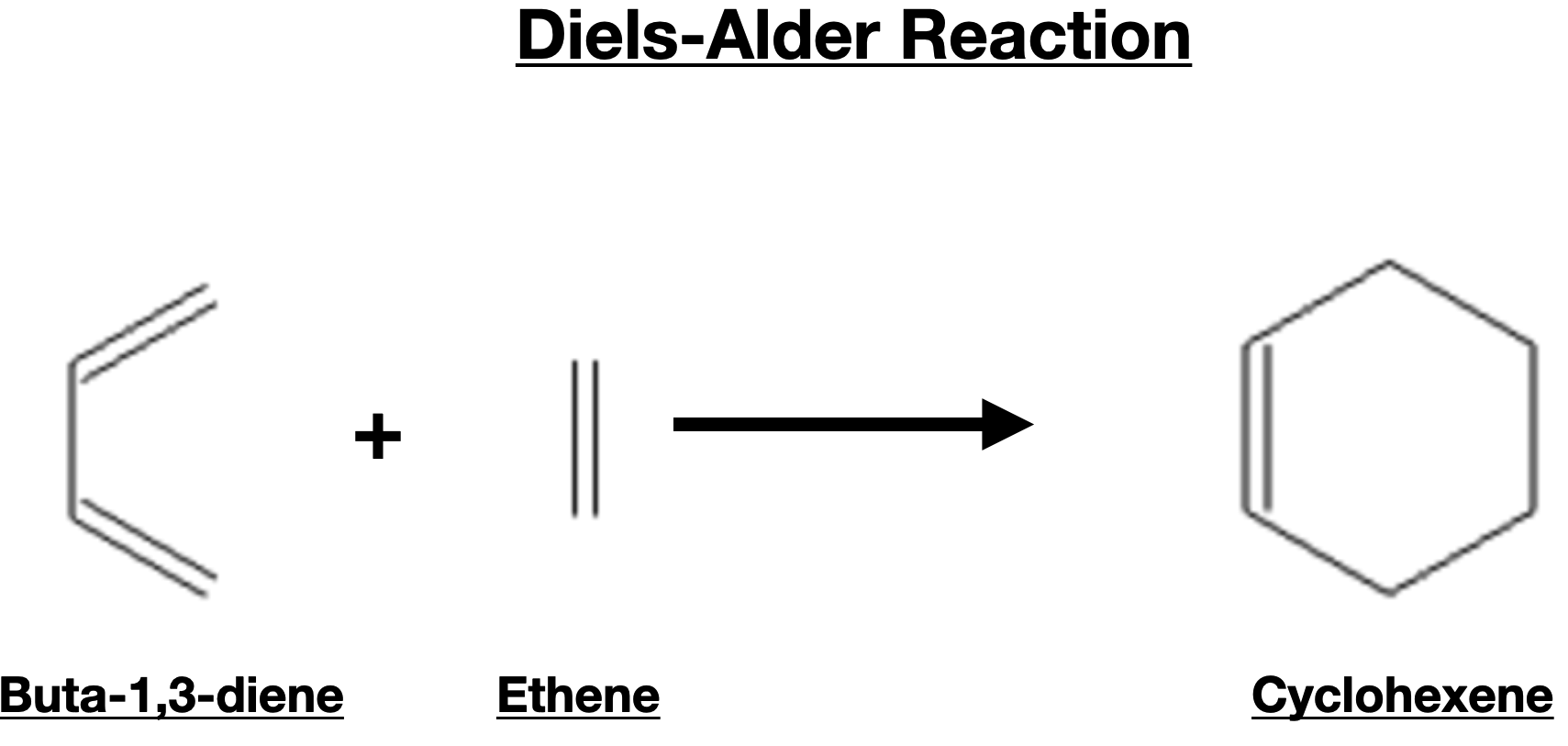
Dienes exhibit unique reactivity due to the presence of multiple double bonds and the possibility of undergoing different types of chemical reactions. One of the most significant reactions involving dienes is the Diels-Alder reaction, which involves the cycloaddition of a diene with a dienophile (an alkene or alkyne). The Diels-Alder reaction is a powerful tool in organic synthesis, allowing the construction of complex cyclic compounds with high regioselectivity and stereoselectivity.
Stereochemistry of Dienes
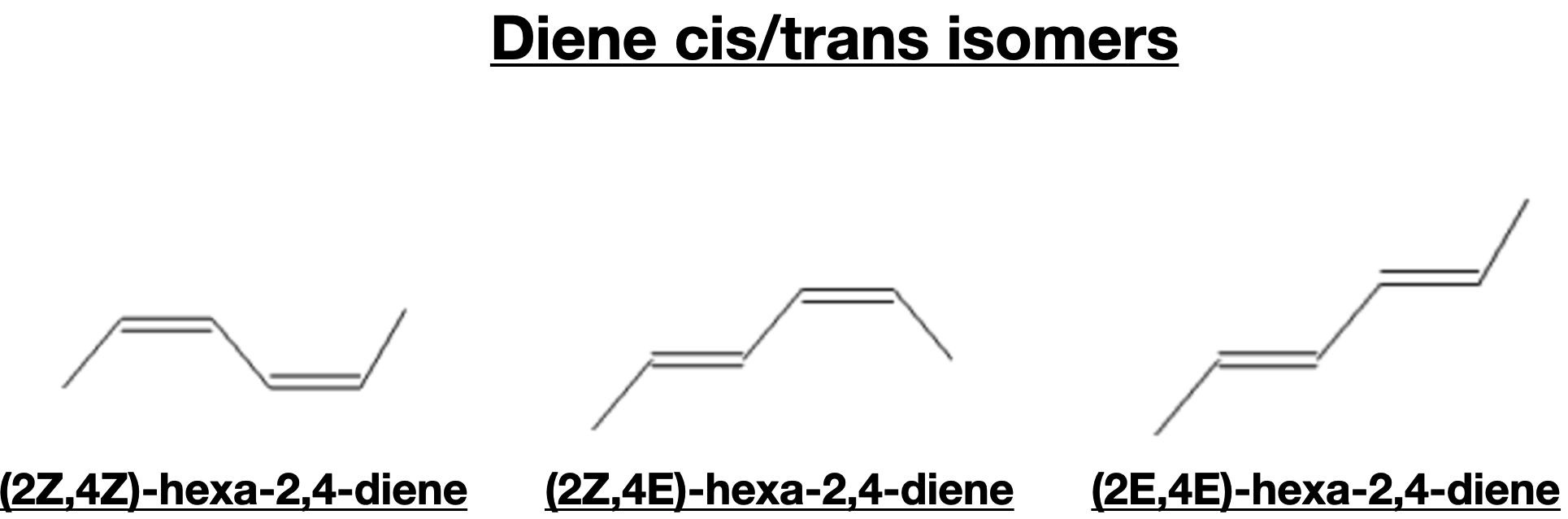
The presence of multiple double bonds in dienes gives rise to interesting stereochemical considerations. Dienes can exhibit cis-trans isomerism, also known as geometric isomerism, based on the arrangement of substituents around the double bonds. The cis-trans isomers have different spatial orientations, leading to distinct chemical properties and reactivity. Understanding cis-trans isomerism is crucial for predicting the outcomes of organic reactions involving dienes.
Cis-trans isomerism, also known as geometric isomerism, is a type of stereoisomerism that occurs in compounds with restricted rotation around a carbon-carbon double bond or a carbon-carbon single bond. In these isomers, the spatial arrangement of substituents attached to the double bond or single bond differs, resulting in distinct chemical properties and reactivity.
In cis isomers, the substituents are located on the same side of the double bond or single bond, while in trans isomers, the substituents are on opposite sides. The cis isomer has a relatively bulky group on each side of the double bond, which can lead to steric hindrance and affect the molecule's reactivity. On the other hand, the trans isomer tends to have lower steric hindrance and can often be more stable.
The E-Z naming nomenclature is used to describe the geometric isomerism of alkenes and other compounds with double bonds. The E (entgegen) configuration indicates that the higher-priority substituents on each carbon of the double bond are on opposite sides, while the Z (zusammen) configuration indicates that the higher-priority substituents are on the same side.
The E-Z naming system follows the Cahn-Ingold-Prelog priority rules, where higher-priority substituents are assigned based on the atomic number of the atoms directly attached to the double bond carbons. By using the E-Z nomenclature, organic chemists can precisely describe the arrangement of substituents around the double bond, which is essential for understanding the reactivity and properties of geometric isomers.
Moreover, dienes can adopt different conformations based on the rotation around single bonds. Conformational analysis using Newman projections allows us to visualize the different arrangements of atoms in the molecule. The stability of different conformations influences the reactivity of dienes in chemical reactions.
Summary
In summary, dienes are a unique and versatile class of organic compounds with two double bonds, offering a wide array of reactivity and stereochemical properties. Their significance in chemical synthesis, polymerization, and other reactions make them an essential topic of study in organic chemistry. By understanding the structure, nomenclature, reactivity, and stereochemistry of dienes, organic chemists can harness their potential in designing efficient and selective synthetic routes for complex molecules.
Test your knowledge
- What is cis/trans conformation? How is it represented in IUPAC naming nomenclature?
- What is an important reaction that dienes are involved in?