Chapter 2 Practice Problems
Chapter 2 Practice Problems
Warm up with naming, conformations, and basic alkane reactivity. Reveal answers after trying each one.
Nomenclature & Properties
-
Name the alkane with formula C₆H₁₄ that has two methyl groups on C-2 of a butane chain.
Answer
2,2-Dimethylbutane. -
Which has the higher boiling point: n-hexane or 2,2-dimethylbutane (shown below)?
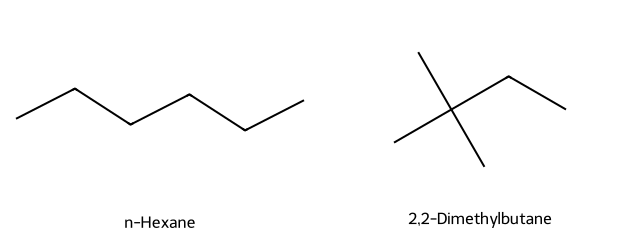
Answer
n-Hexane; less branching → stronger dispersion forces.
Conformational Analysis
-
For butane, which Newman conformer about C2–C3 is lowest in energy: anti, gauche, or eclipsed?
Answer
Anti (methyl groups 180° apart). Gauche is higher; eclipsed is highest. -
In methylcyclohexane, is the equatorial or axial conformation lower in energy? Why?
Answer
Equatorial; it avoids 1,3-diaxial steric interactions.
Reactivity
-
Propane chlorination gives two products. Which is major?
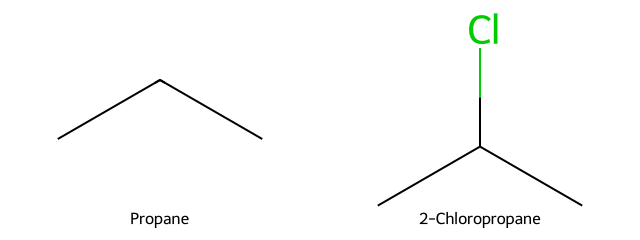
Answer
2-Chloropropane (secondary radical favored over primary). -
Balance the combustion of cyclopentane.
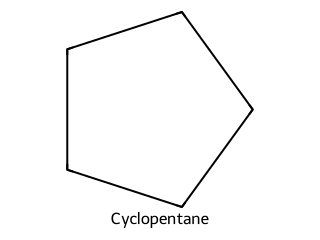
Answer
2 C₅H₁₀ + 15 O₂ → 10 CO₂ + 10 H₂O.