SN1, SN2, E1, E2: Mechanism Guide
SN1, SN2, E1, E2: How to Choose the Mechanism
Substitution (SN1/SN2) and elimination (E1/E2) compete for alkyl halides/alcohol derivatives. Predicting outcomes hinges on substrate, nucleophile/base strength, solvent, and temperature.
Mechanism Snapshots
- SN1: Two-step via carbocation; rate = k[sub]; racemization; rearrangements possible; favored by 3° (some 2°), good leaving group, polar protic solvent, weak/neutral nucleophile.
- SN2: One-step backside attack; inversion; no rearrangement; rate = k[sub][Nu]; favored by 1° (methyl) substrates, strong nucleophile, polar aprotic solvent; hindered substrates slow/blocked.
- E1: Two-step via carbocation; rate = k[sub]; competes with SN1; Zaitsev alkene; possible rearrangements; favored by 3° (some 2°), polar protic solvent, heat.
- E2: One-step, strong base abstracts β-H as LG departs; anti-periplanar geometry required; rate = k[sub][base]; often with 2°/3° + strong (often bulky) base; Hofmann possible with bulky base; no rearrangements.

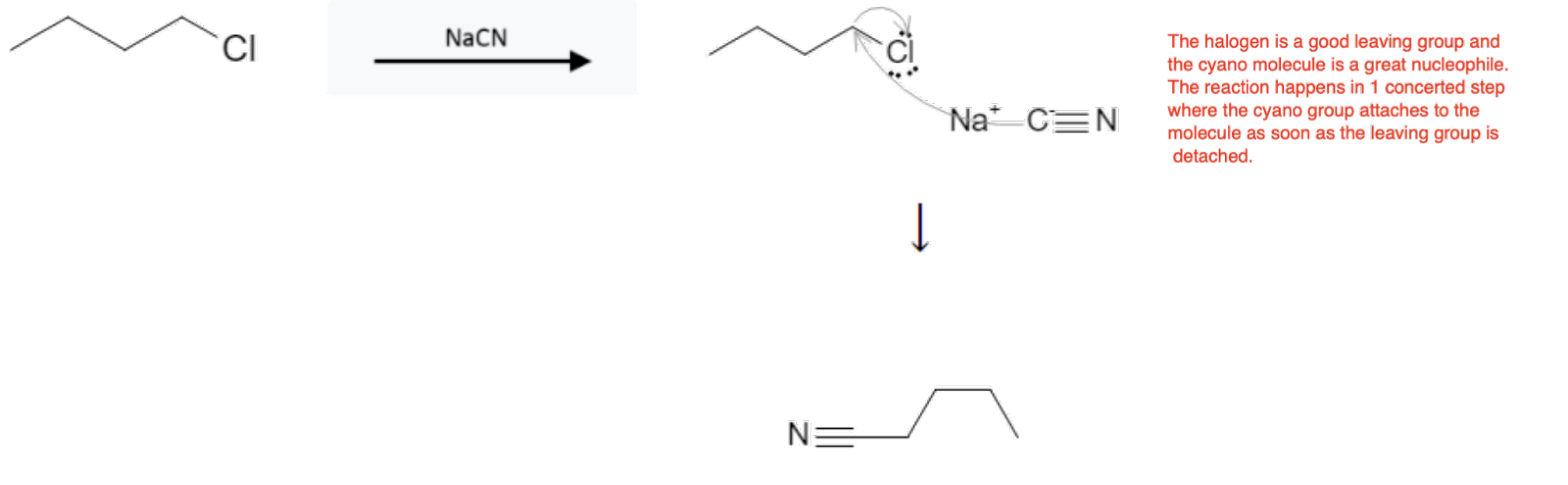

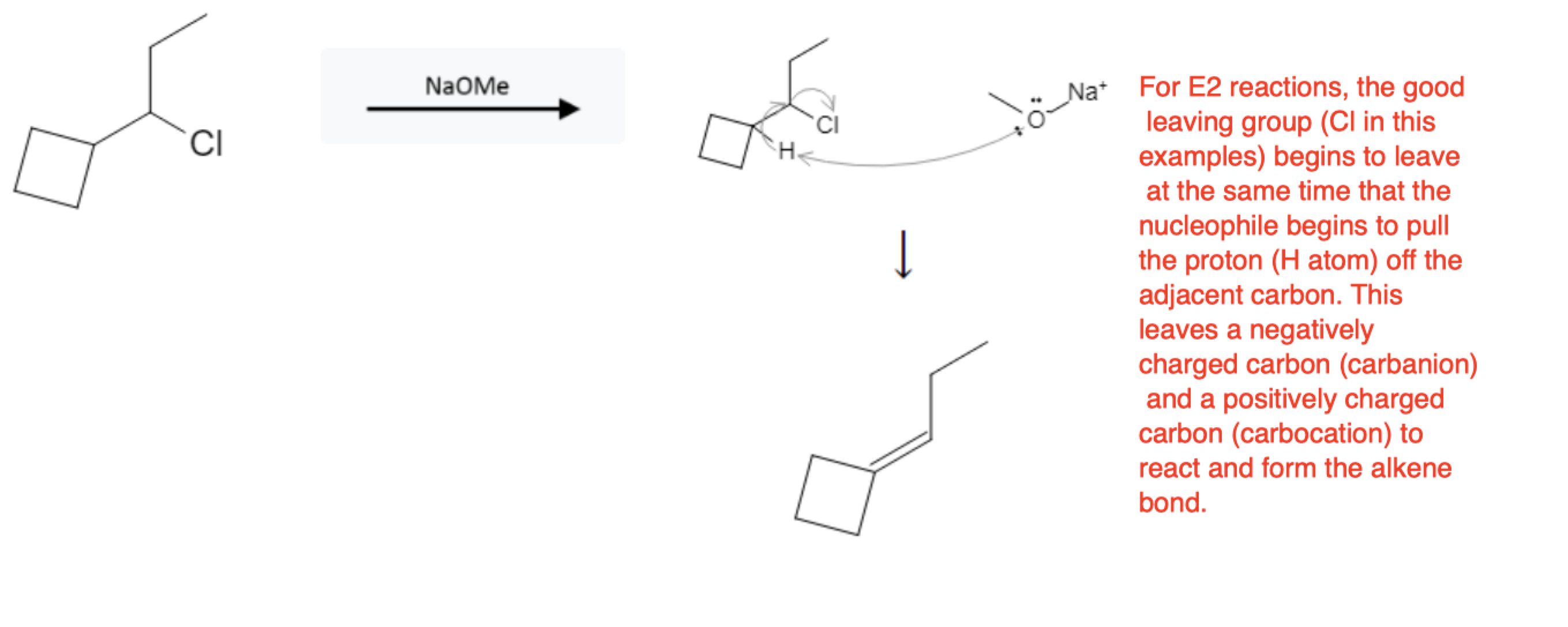
How to Choose
- Substrate: 3° blocks SN2; 1° blocks SN1/E1; 2° is contextual. Allylic/benzylic can do both SN1/SN2; vinyl/aryl generally neither.
- Nucleophile vs base: Strong, unhindered Nu → SN2 (especially 1°); strong bulky base → E2; weak/neutral in protic → SN1/E1 (with 2°/3°).
- Solvent: Protic stabilizes carbocations/ions → SN1/E1; aprotic boosts nucleophiles → SN2/E2.
- Temperature: Higher T often favors elimination (E1/E2) over substitution when pathways compete.
Summary
- SN1/E1 share a carbocation first step (rearrangements, racemization, Zaitsev alkene).
- SN2/E2 are concerted (no rearrangements); SN2 inverts stereochemistry, E2 needs anti geometry.
- Decide by weighing substrate hindrance, nucleophile/base strength, solvent, and temperature.