Reactions of Alkynes
Reactions of Alkynes
Alkynes undergo many additions analogous to alkenes, but each π bond can react, so you can stop at the alkene stage or go all the way to saturated products. Key levers: hydrogenation (partial vs full), hydration (Markovnikov vs anti-Markovnikov), hydrohalogenation, halogenation, and oxidative cleavage.
Hydrogenation
- Full: H₂ with Pd/Pt/Ni reduces alkynes → alkanes (syn overall).
- Partial, cis: Lindlar catalyst (Pd/CaCO₃ or Pd/BaSO₄, Pb(OAc)₂, quinoline) gives cis alkenes.
- Partial, trans: Dissolving metal (Na/NH₃(l)) gives trans alkenes.
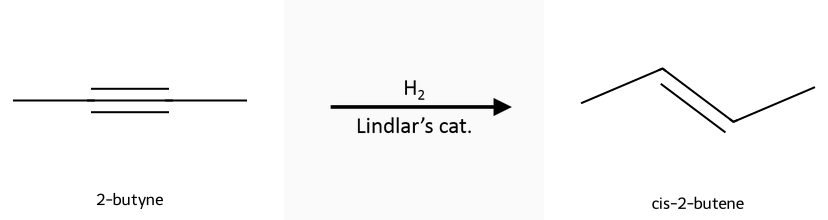
Hydrogenation (H₂, catalyst): Reactant, reagent, product in one row: 2-butyne → cis-2-butene under Lindlar (or trans-2-butene under Na/NH₃). Pd/Pt/Ni with H₂ goes all the way to the alkane. See the full guides on Lindlar reduction and Na/NH₃ reduction.
Hydration
- Markovnikov (Hg²⁺/H₂O/H⁺): Vinyl cation-like intermediate → enol → ketone (acid-catalyzed tautomerization).
- Anti-Markovnikov (Hydroboration–oxidation): 9-BBN or disiamylborane, then H₂O₂/NaOH → enol → aldehyde from terminal alkynes.
- Internal alkynes give ketones regardless of the path (mix if unsymmetrical).
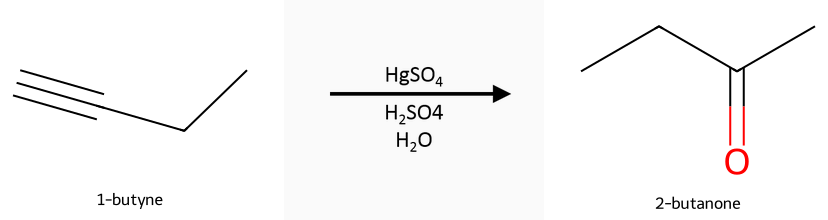
Hydration paths: Reactant, reagent, product: 1-butyne → 2-butanone under HgSO₄/H₂SO₄/H₂O (Markovnikov). Hydroboration–oxidation routes to aldehydes (anti-Markovnikov) on terminal alkynes. Internal alkynes give ketones either way. See oxymercuration hydration and hydroboration–oxidation.
Hydrohalogenation
- HX (HCl, HBr, HI) adds Markovnikov to give vinyl halides (1 equiv) or geminal dihalides (2 equiv).
- Vinyl halide stage can be isolated with 1 equiv HX; excess drives to the dihalide.
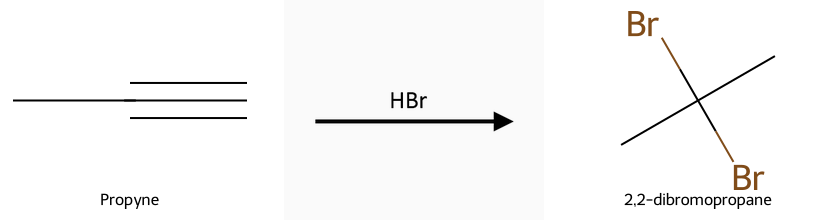
Hydrohalogenation (HX): Reactant, reagent, product: propyne + HX gives the Markovnikov vinyl halide (1 eq) or the geminal dihalide (2 eq). No carbocation shifts, but regiochemistry follows Markovnikov. Full details in the alkyne hydrohalogenation guide.
Halogenation
- X₂ (Br₂ or Cl₂) gives anti addition via a halonium → trans dihaloalkenes (1 equiv) or tetrahalides (2 equiv).
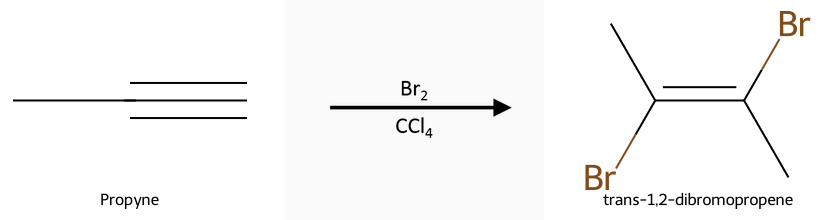
Halogenation (X₂): Reactant, reagent, product: propyne + Br₂/CCl₄ gives the trans dihaloalkene (1 equiv); 2 equivalents drive to the tetrahalide. Mechanistic steps and stereochemistry are covered in the halogenation guide.
Oxidation
- Mild KMnO₄ (cold/neutral) or O₃ (non-oxidative workup): vicinal diketones (or α-keto acids from terminal alkynes).
- Strong KMnO₄ (hot) or O₃/H₂O₂: cleavage to carboxylic acids; terminal carbon → CO₂.
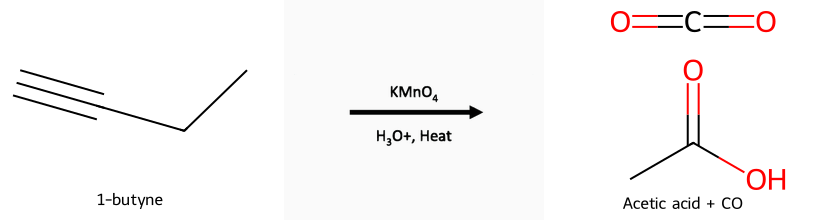
Oxidation/cleavage: Reactant, reagent, product: 1-butyne under strong oxidants (hot KMnO₄ or O₃/H₂O₂) cleaves to acids plus CO₂ from the terminal carbon. Milder oxidants stop at diketones. See the alkyne oxidation guide and ozonolysis guide.
Summary
- Hydrogenation: full (alkane) vs partial (cis or trans alkene).
- Hydration: Hg²⁺ → ketone (Markovnikov); hydroboration–oxidation → aldehyde (anti-Markovnikov) for terminal alkynes.
- Hydrohalogenation: Markovnikov vinyl halide (1 eq) → geminal dihalide (2 eq).
- Halogenation: anti addition to dihaloalkene (1 eq) → tetrahalide (2 eq).
- Oxidation: mild → diketone; strong → cleavage to acids (CO₂ from terminal carbon).