Acid–Base Chemistry of Alcohols and Ethers
Acid-base chemistry is a fundamental concept in organic chemistry. The acid-base properties of alcohols can be analyzed in terms of the Brønsted-Lowry theory. According to this theory, an acid is a substance that donates a proton (H+ ion) and a base is a substance that accepts a proton. In the case of alcohols, they can act as both acids and bases.
Acidity of alcohols
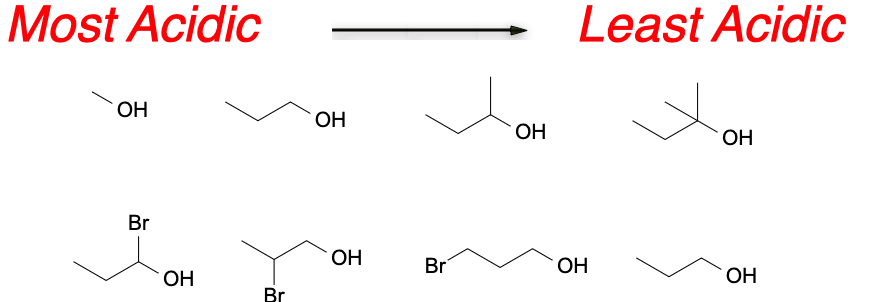
Alcohols are generally weak acids. This is because the hydroxyl group (-OH) in the alcohol molecule is not a good leaving group. However, the acidity of an alcohol is influenced by the electronic effects of the substituents on the carbon atom adjacent to the hydroxyl group. The electron-withdrawing groups, such as halogens or carbonyl groups, increase the acidity of the alcohol, while electron-donating groups, such as alkyl groups, decrease the acidity of the alcohol. This is due to the fact that the electron-withdrawing groups pull electron density away from the hydroxyl group, making it more likely to donate a proton.

Basicity of alcohols
Alcohols can also act as bases by accepting a proton. The basicity of an alcohol is determined by the stability of the corresponding alkoxide ion formed by removing a proton from the hydroxyl group. The more stable the alkoxide ion, the more basic the alcohol. Therefore, the basicity of alcohols follows the reverse trend of their acidity; that is, alkyl groups increase the stability of the alkoxide ion and increase the basicity of the alcohol, while electron-withdrawing groups decrease the stability of the alkoxide ion and decrease the basicity of the alcohol.
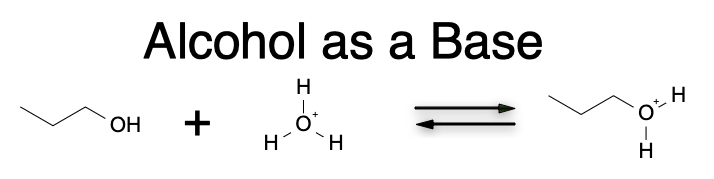
Comparison of acidity of different groups of alcohols
Ethers as Lewis bases
Ethers are also capable of acting as Lewis bases, which means that they can donate a pair of electrons to a Lewis acid. This is due to the presence of lone pairs of electrons on the oxygen atom in the ether molecule. The Lewis basicity of ethers is generally lower than that of alcohols because the oxygen is sandwhiched in between two alkyl groups.
Summary
In summary, alcohols can act as both acids and bases due to the presence of the hydroxyl group in their structure. The acidity of an alcohol is influenced by the electronic effects of the substituents on the adjacent carbon atom. The basicity of an alcohol is determined by the stability of the corresponding alkoxide ion. Ethers are also capable of acting as Lewis bases due to the presence of lone pairs of electrons on the oxygen atom.
Test Your Knowledge:
What is the Brønsted-Lowry theory and how does it apply to alcohols?
How does the electronic effect of substituents on the adjacent carbon atom affect the acidity of alcohols?