Nomenclature and Properties of Alcohols and Ethers
Alcohols and ethers are important functional groups in organic chemistry. They both contain oxygen atoms, but in different arrangements. In this article, we will discuss the nomenclature and properties of alcohols and ethers.
Nomenclature of Alcohols
Alcohols are named by replacing the -e ending of the corresponding alkane with -ol. The longest carbon chain that contains the hydroxyl group (-OH) is selected as the parent chain. The position of the hydroxyl group is indicated by a number, which is assigned to the carbon atom to which it is attached. If there are multiple hydroxyl groups, they are indicated by the prefixes di-, tri-, etc. For example, the alcohol with the chemical formula CH3CH2CH2OH is named propan-1-ol.:
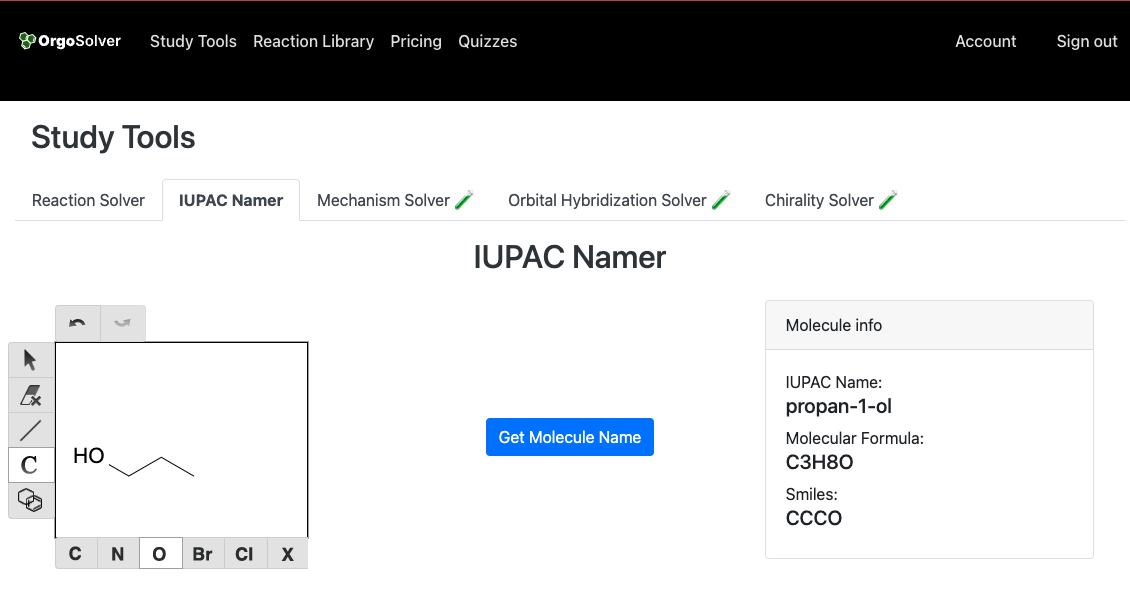
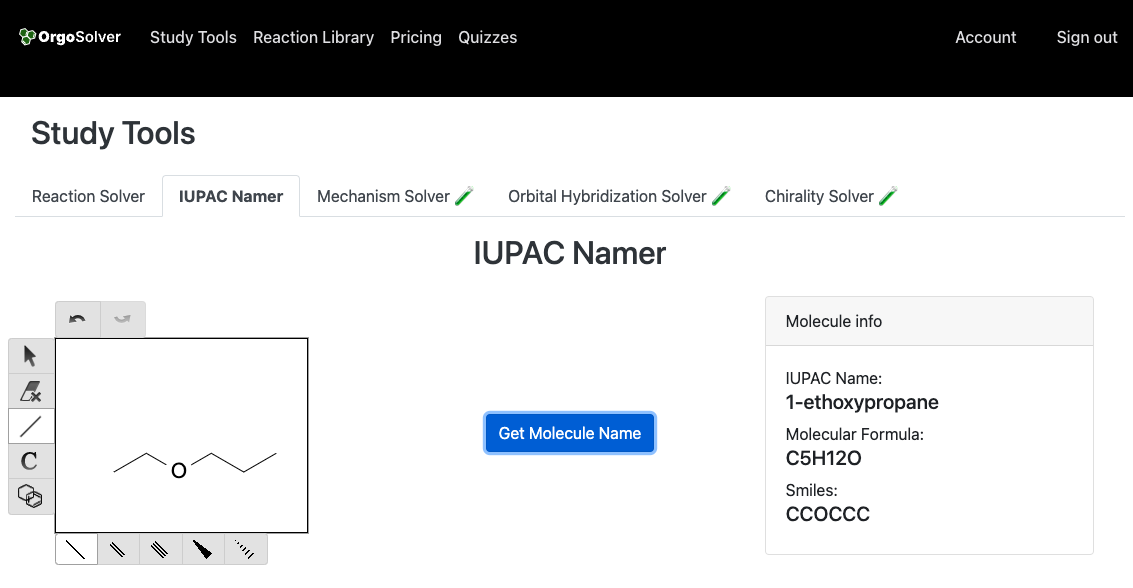
Nomenclature of Ethers
Ethers are named by taking the names of the two alkyl or aryl groups that are attached to the oxygen atom and adding the word ether. The two groups are listed in alphabetical order. The prefix di- is used if there are two identical groups. The two alkyl groups are joined with the word "oxy". For example, this molecule is named 1-ethoxypropane.
Properties of Alcohols
Alcohols are polar molecules due to the presence of the hydroxyl group, which is a polar functional group. They can form hydrogen bonds with other polar molecules, which makes them soluble in water. The boiling points of alcohols increase with increasing molecular weight, as well as with the increasing number of hydroxyl groups. Alcohols can also undergo reactions such as dehydration, oxidation, and esterification.
Properties of Ethers
Ethers are relatively unreactive due to their lack of polar functional groups. They have lower boiling points compared to alcohols of similar molecular weight, as they do not form hydrogen bonds with each other. Ethers are also generally insoluble in water.
Check out our Organic Compound Namer to draw any molecule and get the IUPAC name!
Summary
Alcohols and ethers are two important functional groups in organic chemistry. Alcohols contain a hydroxyl group (-OH), which makes them polar and able to form hydrogen bonds. Ethers lack polar functional groups and are generally unreactive. Both alcohols and ethers can be named using specific nomenclature rules.
Test Your Knowledge:
What is the nomenclature rule for naming alcohols? Provide an example.
What property do alcohols have that ethers lack, and how does this property affect their solubility in water?