Structure and Basicity of Amines
Amines are organic compounds that contain a nitrogen atom bonded to one or more alkyl or aryl groups. They are considered to be basic due to the presence of the nitrogen lone pair electrons. These lone pair electrons can interact with protons (H+) from acids to form a coordinate covalent bond, resulting in the formation of an ammonium ion.
Basicity is a measure of the ability of a substance to donate a pair of electrons to a proton. In the case of amines, the basicity is related to the availability of the nitrogen lone pair electrons. The basicity of amines depends on several factors including the electronic effects of the substituents on the nitrogen atom and the steric hindrance around the nitrogen atom.
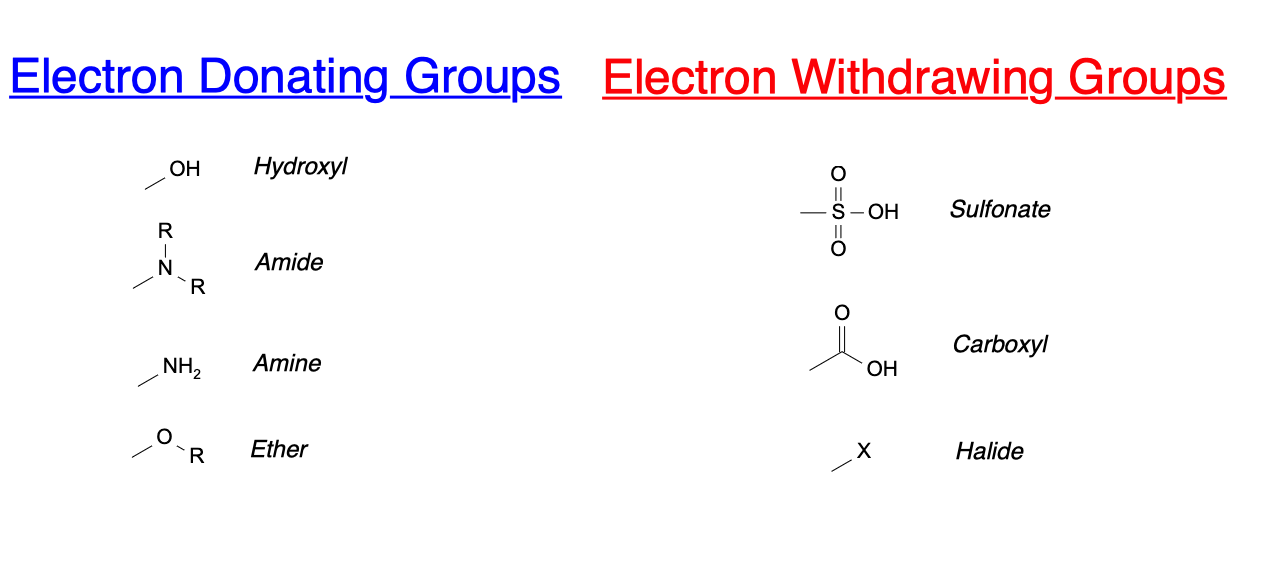
The electronic effects of the substituents can be either inductive or resonance effects. Inductive effects are due to the electronegativity difference between the nitrogen atom and the substituents. If the substituent is electron-withdrawing, it will decrease the electron density on the nitrogen atom, making it less basic. Conversely, if the substituent is electron-donating, it will increase the electron density on the nitrogen atom, making it more basic.
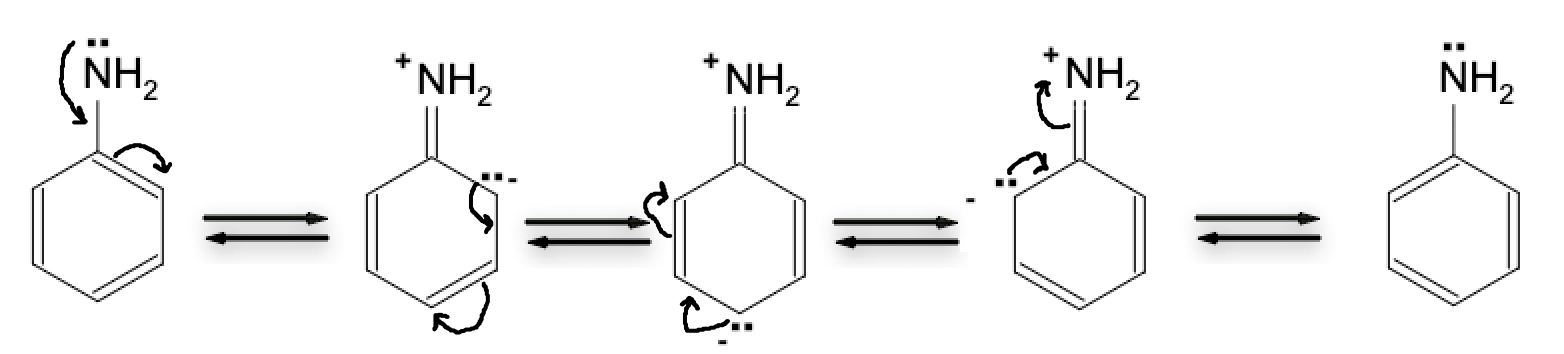
Resonance effects arise from the delocalization of the nitrogen lone pair electrons into a pi bond with a neighboring atom. The greater the number of resonance structures formed, the less basic (lower pKa) the molecule is. Aniline (shown below) has 5 resonance structures, making it a much weaker base compared to ammonia or methylamine.
Aniline
Steric hindrance around the nitrogen atom can also affect the basicity of amines. When there are bulky substituents near the nitrogen atom, it can hinder the interaction of the nitrogen lone pair electrons with protons, resulting in a decrease in basicity.
The basicity of amines can be measured using their pKa values, which is the negative logarithm of the acid dissociation constant. Amines with lower pKa values are more basic and have a higher tendency to donate electrons to form a coordinate covalent bond with protons. Examples of amines with varying basicity include:
Ammonia (NH3): pKa = 9.25
Methylamine (CH3NH2): pKa = 10.64
Ethylamine (C2H5NH2): pKa = 10.75
Aniline (C6H5NH2): pKa = 4.63
Summary
The basicity of amines depends on the electronic effects of substituents on the nitrogen atom, resonance effects, and steric hindrance around the nitrogen atom. The basicity can be measured using their pKa values. The lower the pKa, the weaker the base.
Test Your Knowledge:
Does a greater number of resonance structures result in a stronger base?
How does the electronic effect of substituents adjacent to the nitrogen atom affect the bascicity of amines?