Nomenclature and Classification of Amines
Amines and amides are important classes of nitrogen-containing compounds that are widely used in various fields such as medicine, agriculture, and industry. In this article, we will discuss the nomenclature and properties of amines and amides.
Nomenclature of Amines:
Amines are organic compounds that contain nitrogen as the primary or secondary functional group. Amines are named based on the number of alkyl or aryl groups attached to the nitrogen atom. In primary amines, the -NH2 group is attached to a carbon atom, and the name of the compound is derived by adding the prefix "amino" to the name of the alkane from which the amine is derived. In secondary amines, there are two alkyl or aryl groups attached to the nitrogen atom, and the name of the compound is derived by adding the prefix "N-" to the name of the alkane from which the amine is derived. Tertiary amines have three alkyl or aryl groups attached to the nitrogen atom, and their names are derived by adding the prefixes "N,N-dimethyl," "N,N-diethyl," etc., to the name of the amine.
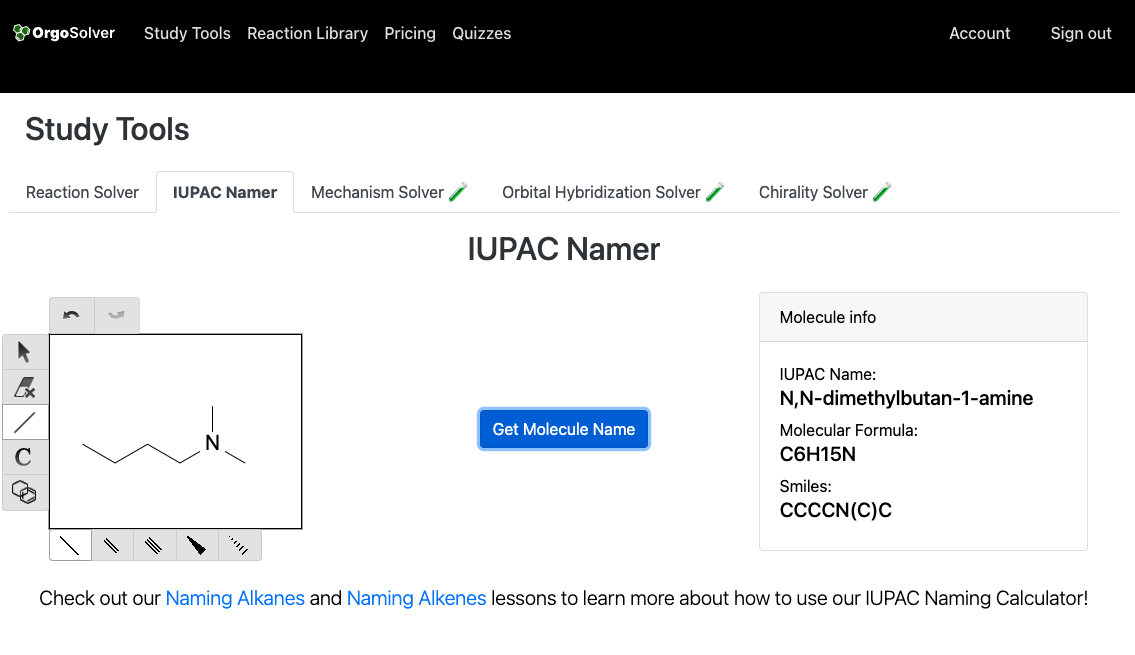
Nomenclature of Amides:
Amides are organic compounds that contain a carbonyl group (C=O) attached to an amine (-NH2) or an ammonia (NH3) molecule. The naming of amides is based on the name of the parent carboxylic acid. In the IUPAC system, the suffix "-amide" is added to the name of the carboxylic acid to form the name of the amide. For example, the amide derived from acetic acid is called acetamide.
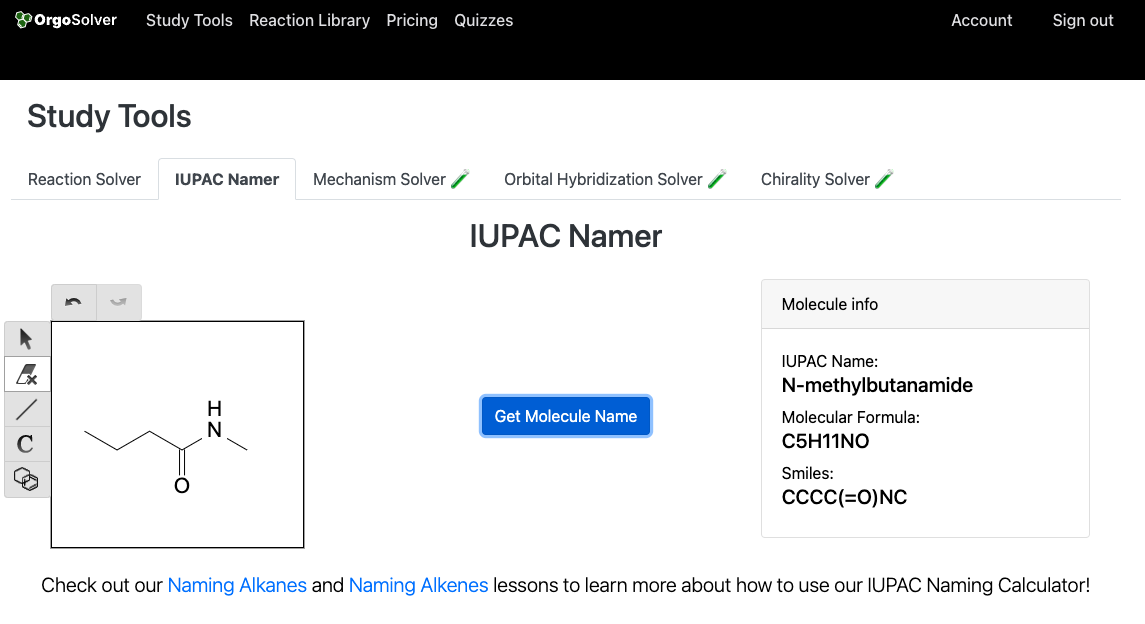
Properties of Amines:
Amines are weak bases due to the presence of the lone pair of electrons on the nitrogen atom. Amines react with acids to form salts, a process known as acid-base reactions. Amines also undergo nucleophilic substitution reactions with alkyl halides to form alkyl amines. In addition, amines can undergo oxidation reactions to form nitro compounds or amine oxides.
Properties of Amides:
Amides are weakly basic due to the presence of the lone pair of electrons on the nitrogen atom. Amides are less basic than amines due to the presence of the carbonyl group, which withdraws electrons from the nitrogen atom. Amides can act as hydrogen bond acceptors due to the presence of the carbonyl group. Amides can also undergo hydrolysis reactions under acidic or basic conditions to form carboxylic acids or amines, respectively.
Check out our Organic Compound Namer to draw any molecule and get the IUPAC name!
Summary
Amines and amides are important classes of nitrogen-containing compounds with a wide range of applications. The nomenclature and properties of these compounds are determined by the number of alkyl or aryl groups attached to the nitrogen atom and the presence of a carbonyl group in the case of amides. Amines are weak bases and can undergo acid-base reactions and nucleophilic substitution reactions, while amides are weakly basic and can act as hydrogen bond acceptors and undergo hydrolysis reactions.
Test Your Knowledge:
What is the IUPAC nomenclature for primary amines, and how is it derived?
How do amines and amides differ in their basicity, and what factors contribute to these differences?