Synthesis and Reactions of Amines and Amides
Alcohols and ethers are important functional groups in organic chemistry that can be synthesized through various methods and undergo a wide range of reactions. In this lesson, we will discuss the synthesis and reactions of alcohols and ethers.
Synthesis of Amines:
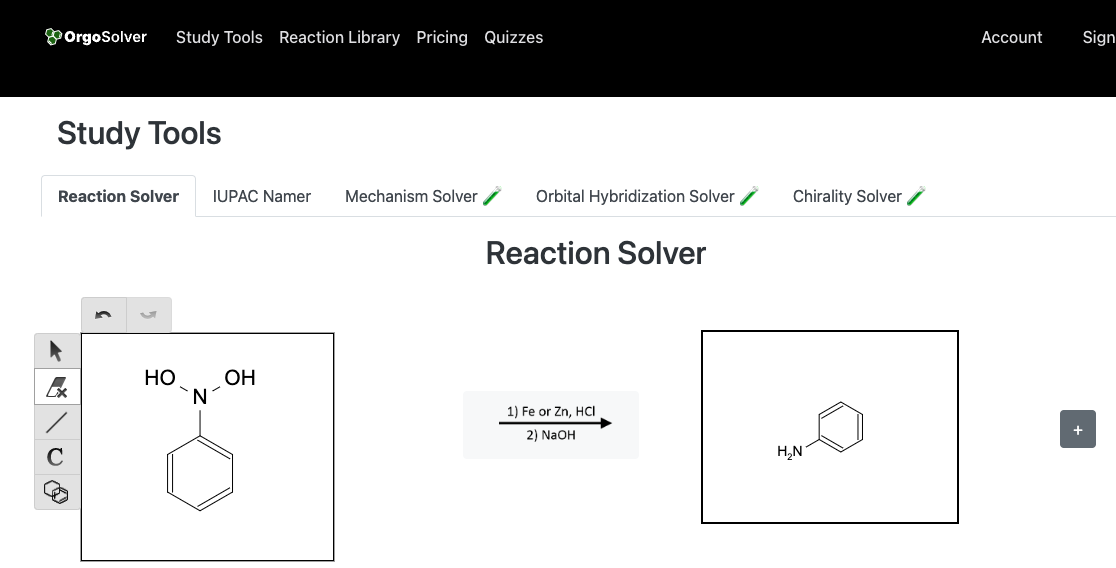
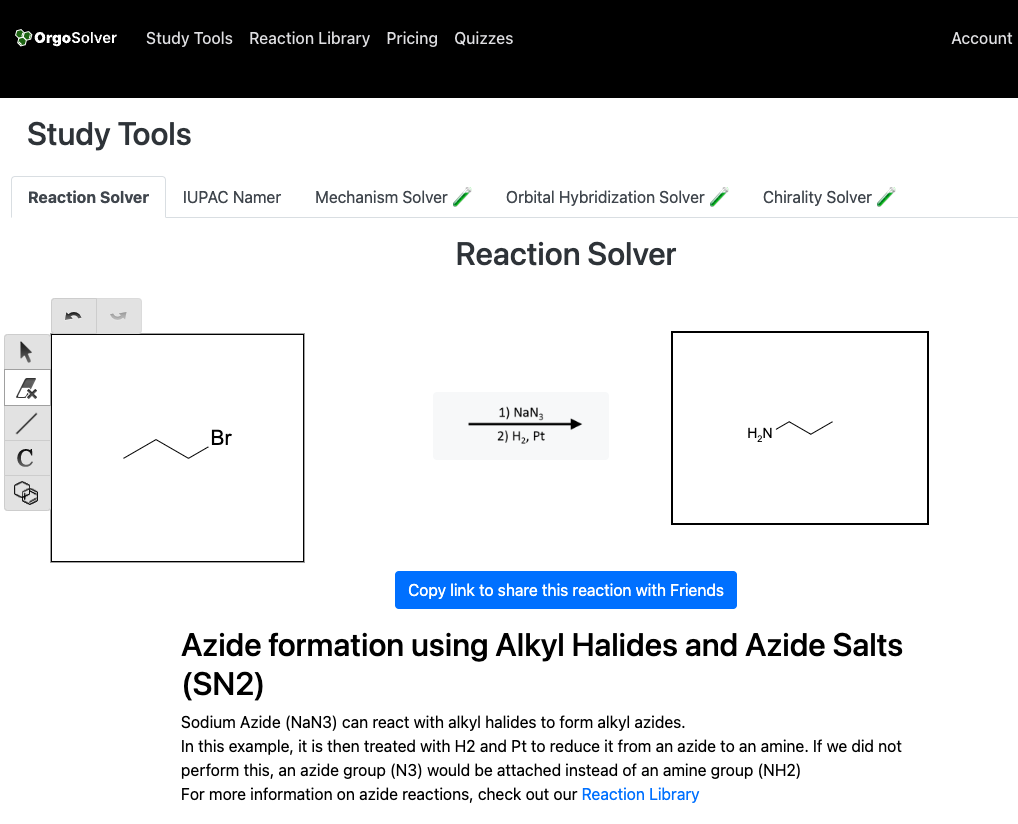
Amines can be synthesized by a variety of methods, such as the reduction of nitro compounds, Gabriel synthesis, reductive amination, and nucleophilic substitution. Reduction of nitro compounds is a widely used method for the preparation of amines. In this method, nitro compounds are reduced to amines by using reducing agents like SnCl2, Fe, or H2/Pt. Gabriel synthesis involves the reaction of potassium phthalimide with an alkyl halide to form a phthalimide salt, which is then hydrolyzed to give the desired amine. Reductive amination is another important method for the synthesis of amines, which involves the reaction of carbonyl compounds with ammonia or primary amines, followed by reduction. Nucleophilic substitution of alkyl halides with ammonia or amines is also a useful method for the synthesis of amines.
Reduction of Nitro compounds
Nucleophillic substitution
Reactions of Amines:
Amines can undergo a variety of reactions such as nucleophilic substitution, oxidation, and alkylation. In nucleophilic substitution reactions, the lone pair of electrons on the nitrogen atom of the amine acts as a nucleophile and attacks an electrophilic center. Amines can also be oxidized to form N-oxides, nitroso compounds, and nitro compounds. Alkylation of amines is another important reaction, which involves the addition of an alkyl group to the nitrogen atom of the amine. Amines are typically listed as reagents in organic chemistry:
Amines as reagents
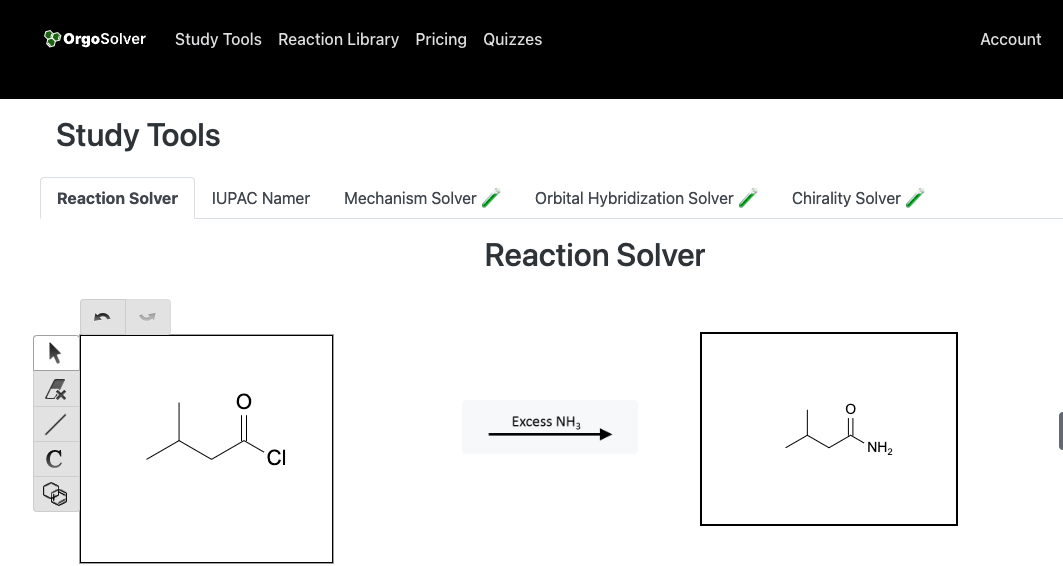
Synthesis of Amides:
Amides can be synthesized by the reaction of carboxylic acids with amines. This reaction is known as the amidation reaction and is typically catalyzed by an acid catalyst like H2SO4. Another important method for the synthesis of amides is the reaction of acid chlorides or acid anhydrides with amines.
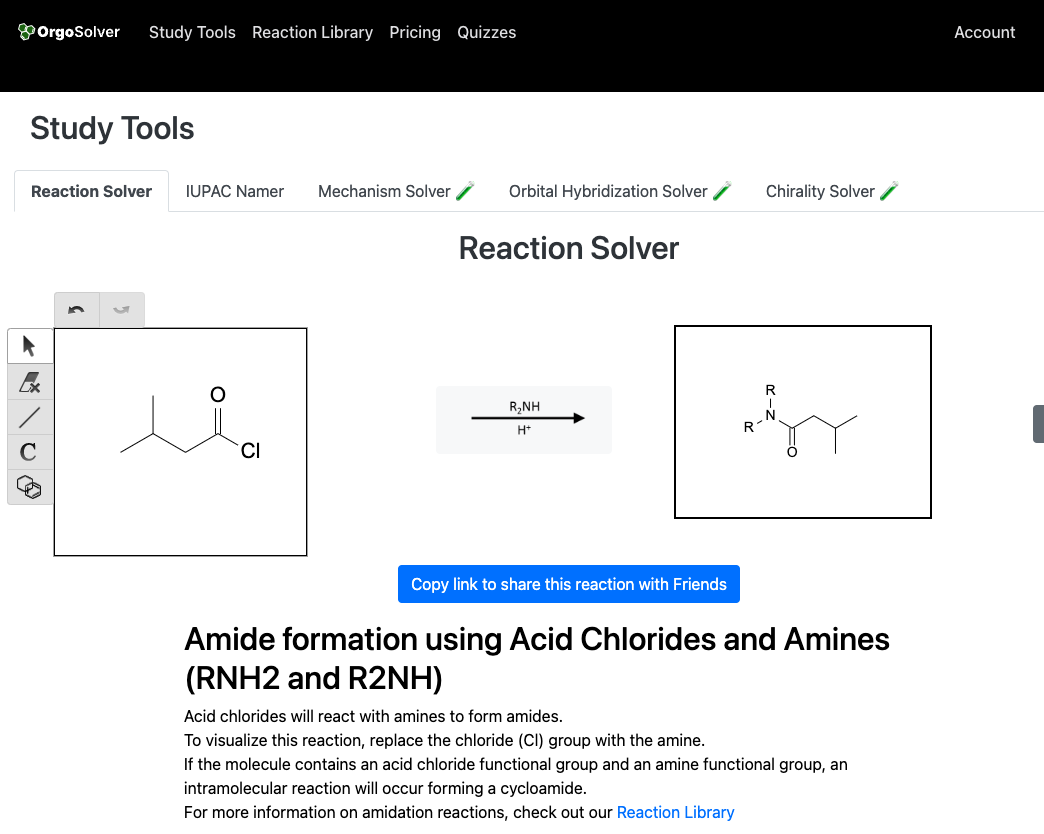
Reactions of Amides:
Amides can undergo a variety of reactions such as hydrolysis, reduction, and acylation. Hydrolysis of amides involves the cleavage of the C-N bond of the amide by water or an acid, resulting in the formation of a carboxylic acid and an amine. Reduction of amides can be achieved by using reducing agents like LiAlH4, resulting in the formation of amines. Acylation of amides involves the addition of an acyl group to the nitrogen atom of the amide.
Summary
Amines and amides are important classes of organic compounds that have a wide range of applications in industry and medicine. The synthesis and reactions of amines and amides are of great importance to organic chemists, and the various methods and reactions discussed in this lesson provide a solid foundation for further study.
Visit our Reaction Solver to draw any molecule, select your reagent, and get an answer!
Test Your Knowledge:
What is the amidation reaction and how is it typically catalyzed?
How can amides be reduced to form amines?