Synthesis and reactions of carboxylic acids, esters, and anhydrides
Carboxylic acids and their derivatives, including esters and anhydrides, are an important class of organic compounds with a wide range of applications in various fields such as pharmaceuticals, perfumes, and plastics. In this article, we will explore the synthesis and reactions of carboxylic acids, esters, and anhydrides.
Synthesis of carboxylic acids:
Carboxylic acids can be synthesized through various methods, including the oxidation of primary alcohols and aldehydes, the hydrolysis of nitriles, and the carboxylation of Grignard reagents. The most common method of preparing carboxylic acids is the oxidation of primary alcohols using an oxidizing agent such as potassium permanganate or chromic acid.
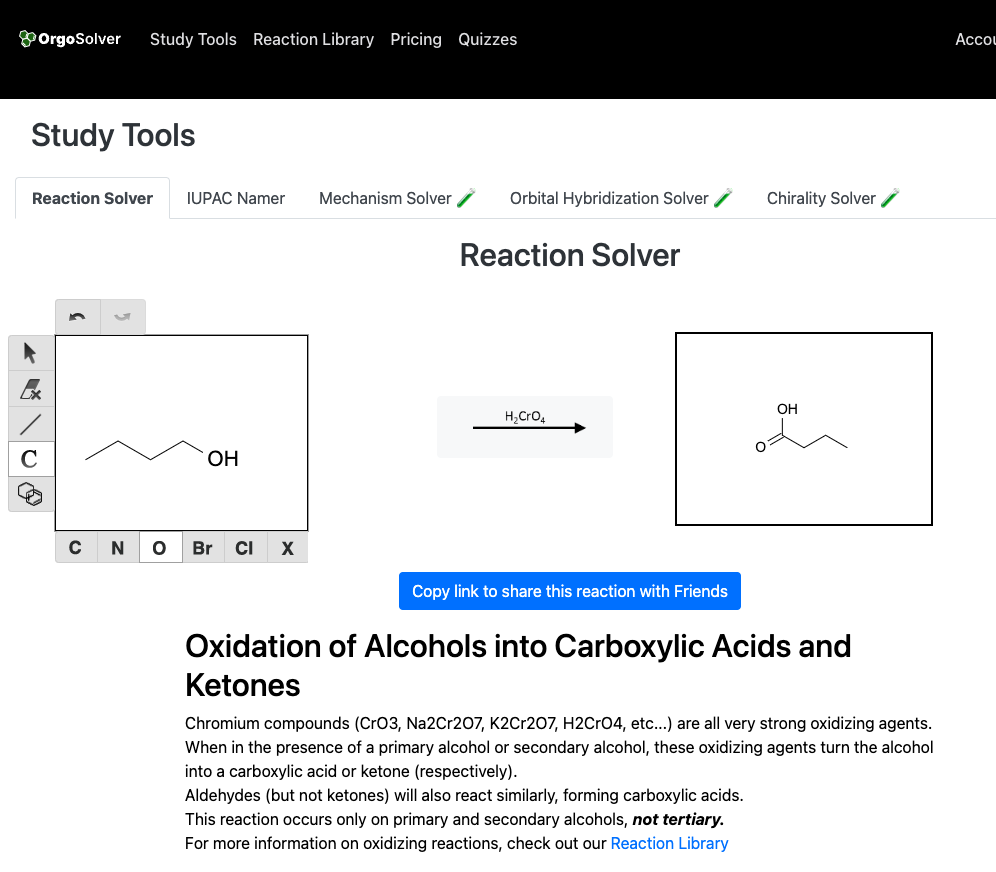
Synthesis of esters:
Esters can be synthesized through the reaction of a carboxylic acid with an alcohol, catalyzed by an acid catalyst. This reaction is known as esterification. The reaction produces an ester and water as a byproduct.
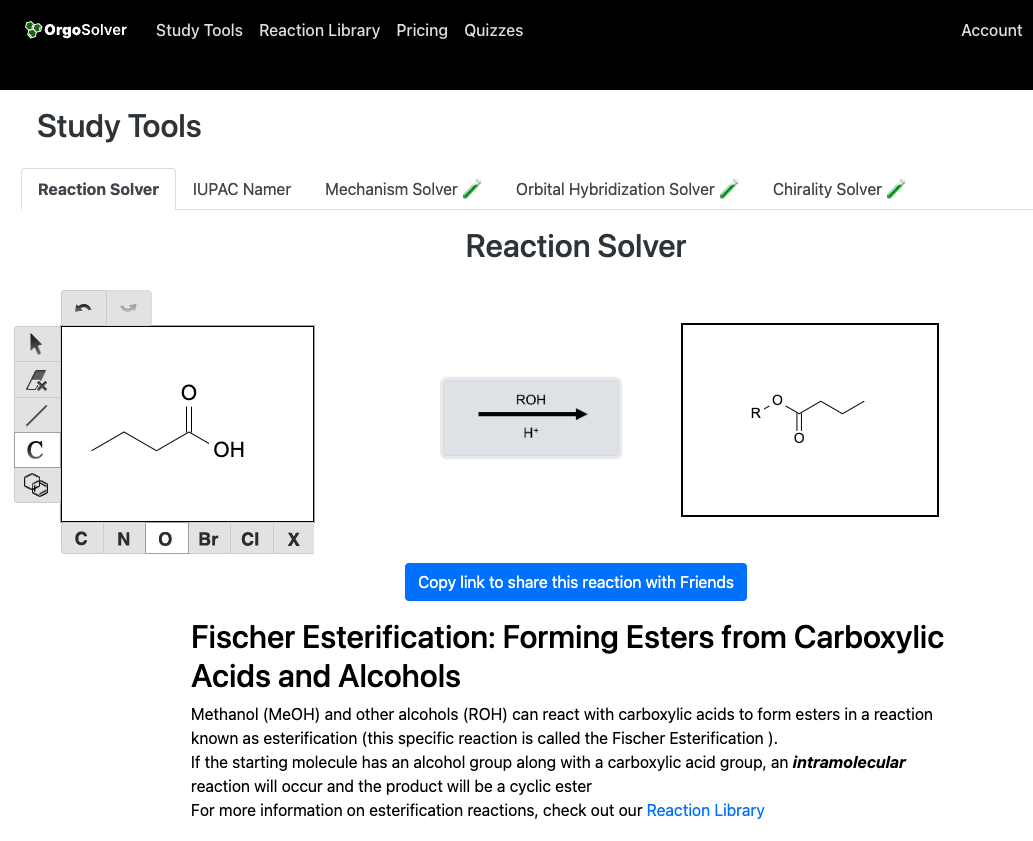
Synthesis of anhydrides:
Anhydrides are formed by the condensation of two carboxylic acid molecules, catalyzed by various agents such as sulfuric acid, phosphoric acid, or acetic anhydride. This reaction is called the dehydration of carboxylic acids.
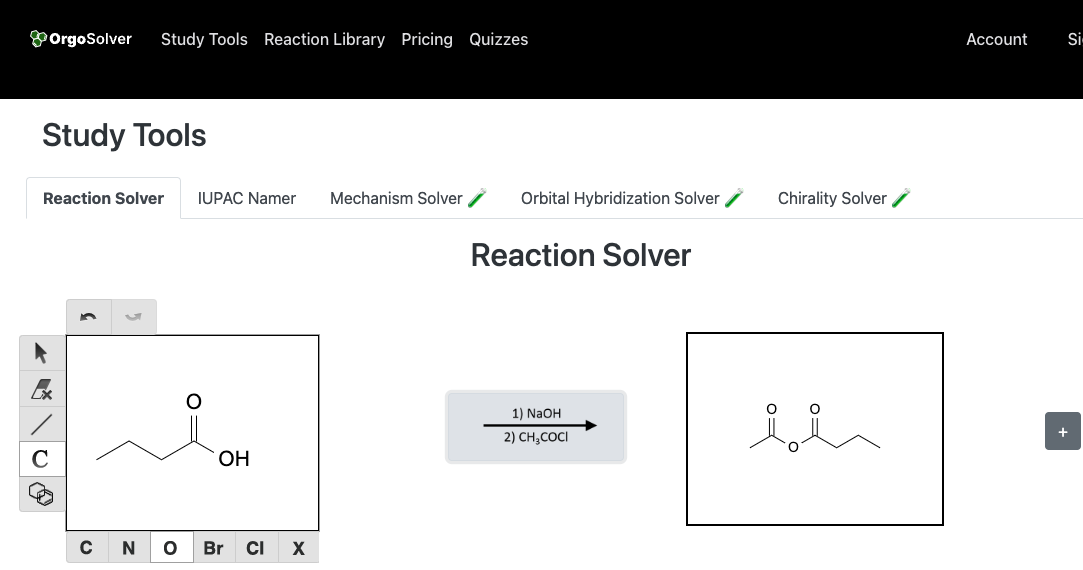
Reactions of carboxylic acids:
Carboxylic acids can undergo various reactions such as nucleophilic substitution, acid-base reactions, and oxidation. One of the most common reactions of carboxylic acids is their reaction with a strong base to form a carboxylate salt and water. They can also be oxidized to form carbon dioxide and water.
Reactions of esters:
Esters can undergo hydrolysis in the presence of a strong acid or base to produce a carboxylic acid and an alcohol. They can also undergo transesterification, which is the exchange of one alcohol group for another. Esters can be reduced to form aldehydes or alcohols as well.
Reactions of anhydrides:
Anhydrides can undergo hydrolysis to form two carboxylic acid molecules. They can also be used as an acylating agent in various reactions such as the Friedel-Crafts acylation reaction.
Summary
Carboxylic acids, esters, and anhydrides are important organic compounds with a wide range of applications. They can be synthesized through various methods, and they undergo different reactions depending on the functional group present. Understanding their synthesis and reactivity is crucial for the development of new materials and drugs.
Visit our Reaction Solver to draw any molecule, select your reagent, and get an answer!
For a complete list of carboxylic acids, esters, and anhydrides reactions used in Organic Chemistry 1, check out our Reaction Library!
Test Your Knowledge:
What is the most common method of preparing carboxylic acids?
What is the name of the reaction that produces an ester and water as a byproduct?