Acid Chloride Reactions: Alcohol formation using Grignard Reagents
Grignard reagents are strong, basic nucleophiles that add to acid chlorides twice. The first equivalent performs nucleophilic acyl substitution: chloride leaves with magnesium to reveal a ketone. Because that ketone is far more electrophilic toward RMgBr than the original acid chloride, a second equivalent immediately adds to form a tertiary magnesium alkoxide. A cold, acidic workup (H₃O⁺) protonates the alkoxide and unveils the tertiary alcohol while magnesium salts partition into the aqueous layer.
Planning-wise, assume you will reach the tertiary alcohol unless you switch to a softer reagent such as a Gilman cuprate (R₂CuLi) or a Weinreb amide. Water, protic functional groups, and carbonyls elsewhere in the molecule must be protected or staged to survive the Grignard.
Quick Summary
- Reagents/conditions: Acid chloride, ≥2.0 eq RMgBr dissolved in rigorously dry Et₂O or THF, −78 → 0 °C, inert atmosphere; then cold dilute H₃O⁺ to protonate the alkoxide and dissolve Mg salts.
- Outcome: R′–COCl + 2 RMgBr → R′–C(OMgBr)R₂ (tertiary alkoxide) → H₃O⁺ → tertiary alcohol R′–C(OH)R₂ with MgBrCl/H₂O byproducts.
- Mechanism: Nucleophilic acyl substitution generates a ketone, then a second nucleophilic addition forms the tertiary alkoxide; final protonation liberates the alcohol.
- Stereochemistry: Attack on the planar ketone creates a new stereocenter; in the absence of chiral induction, the tertiary alcohol is racemic.
- Contrasts: Gilman reagents (R₂CuLi) stop at the ketone; LiAlH₄ reduces acid chlorides to primary alcohols; Weinreb amides allow a ketone to be isolated.
- Common pitfalls: Budgeting only one equivalent of RMgBr, exposing protic/acidic functionalities, or assuming the ketone can be isolated under these basic, nucleophilic conditions.
Mechanism — Five Steps (Double Addition, Then Protonation)
Each frame comes directly from the RDKit builder used in the Mechanism Solver. Seafoam-teal (Color 1) traces the carbon fragment(s) delivered by RMgBr; no Color 2 highlight is required for this mechanism.
Mechanistic Checklist
- Two equivalents of RMgBr are mandatory: the first forms the ketone, the second converts it to the tertiary alkoxide.
- The ketone intermediate is transient because it is more electrophilic than the starting acid chloride.
- Competent protecting groups are necessary for any –OH, –NH, acidic C–H, or carbonyl functions elsewhere in the molecule.
- Protonation is strictly at the alkoxide stage; the H₃O⁺ workup is separate from the carbon–carbon bond-forming steps.
- Expect racemic mixtures at the newly formed tertiary center unless a chiral auxiliary/catalyst is used.
Worked Examples
Reactant
Reagent
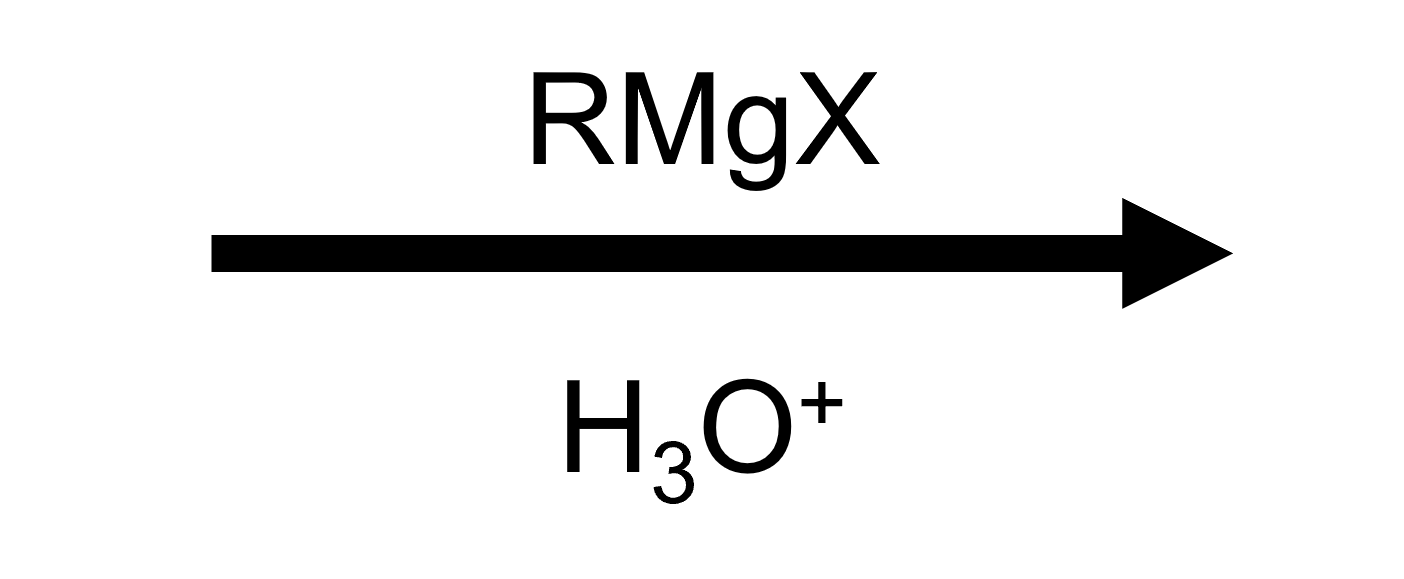
Product
2-methylpropan-2-ol.
Reactant
Reagent
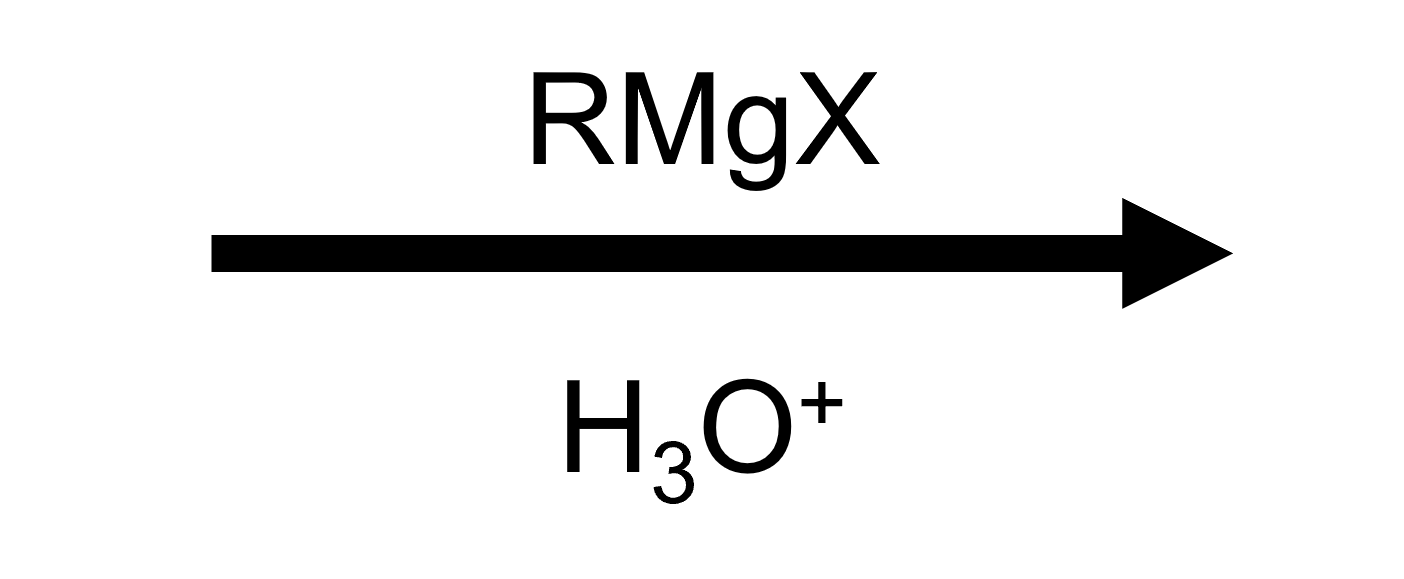
Product
Triphenylmethanol.
Reactant
Reagent
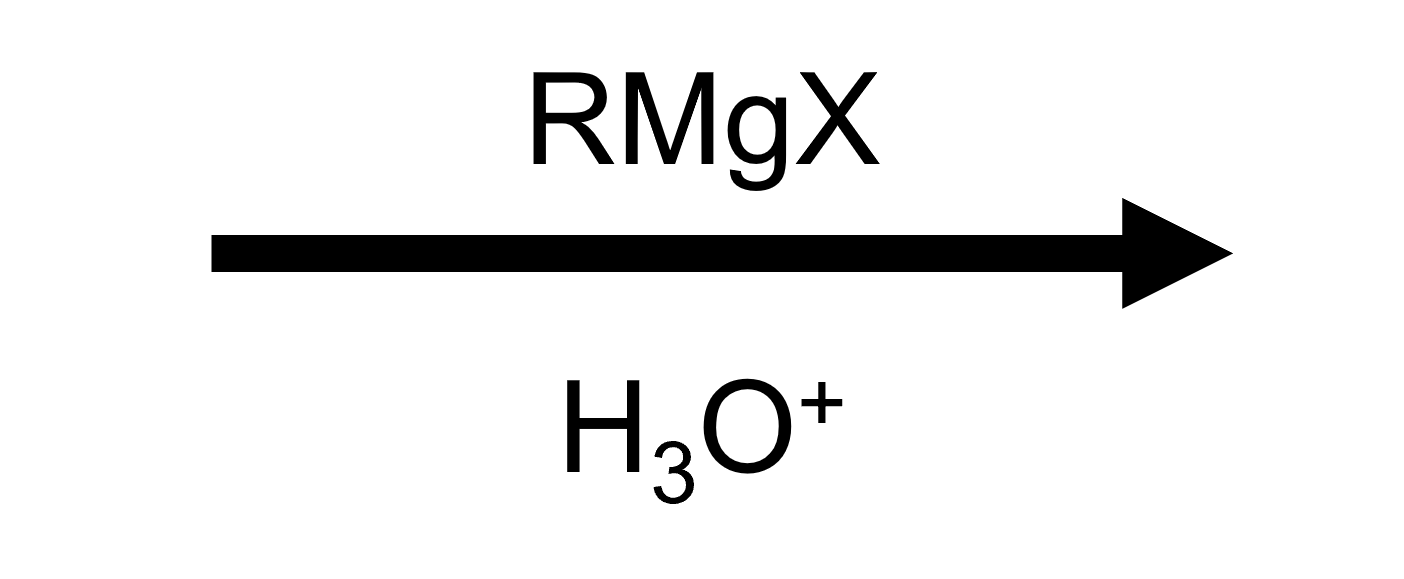
Product
3-methylpentan-3-ol.
Each worked example reuses the same SMILES that feed the Mechanism Solver, so what you see here is exactly what appears in the interactive tool.
Scope & Limitations
- Works well: Aliphatic, benzylic, and aromatic acid chlorides with alkyl, aryl, vinyl, or allylic Grignards in dry ether or THF at sub-ambient temperatures.
- Sensitive: Acid chlorides bearing protic groups (–OH, –NH, –CO₂H) or embedded carbonyls will be attacked unless protected; even aldehydes and ketones elsewhere on the molecule will react faster than the acid chloride.
- Steric effects: Bulky acid chlorides or very hindered Grignards react sluggishly; cooling slows down decomposition but also slows the addition.
- Chemoselectivity: RMgBr ignores poor leaving groups like OR in esters and amides under these conditions, but any carbonyl with a better leaving group (acid anhydrides, acid bromides) will also react.
Practical Tips
- Flame-dry or oven-dry all glassware, maintain inert gas (N₂/Ar), and keep ether/THF scrupulously dry; water destroys RMgBr instantly.
- Add the acid chloride slowly to the cold Grignard to moderate heat and keep the first addition faster than any side reactions.
- Plan a two-stage quench: a sacrificial electrophile (EtOAc or i-PrOH) can consume any leftover RMgBr before you add the cold H₃O⁺.
- Track equivalents carefully; triggering the reaction with <2 eq RMgBr almost guarantees a mixture of ketone and tertiary alcohol.
Exam-Style Summary
Acid chloride + ≥2 eq RMgBr gives a tertiary alkoxide that H₃O⁺ protonates to the tertiary alcohol; the ketone intermediate is too reactive to isolate. Need the ketone? Use R₂CuLi or a Weinreb amide. Need a primary alcohol? Reduce the acid chloride with LiAlH₄ instead.
Pitfalls to watch for:
- Predicting a ketone product after RMgBr is almost always wrong—assume the tertiary alcohol unless the reagent is changed.
- Forgetting to protect protic groups or carbonyls elsewhere on the molecule invites unplanned RMgBr additions.
- Assuming stereoselectivity: without a chiral reagent or auxiliary, the new tertiary center is racemic.
FAQ
Why can’t I stop after the first addition to isolate the ketone?
The ketone formed after chloride departure is more electrophilic than the starting acid chloride, so the second equivalent of RMgBr reacts even faster. Switch to a Gilman (R₂CuLi) or convert the acid chloride into a Weinreb amide if you need a controllable single addition.
What happens if I only use one equivalent of RMgBr?
You’ll generate a mixture: some ketone (from the first addition) and unreacted acid chloride. The ketone still consumes RMgBr rapidly, so overall yield of the tertiary alcohol plummets and purification becomes messy.
Do I have to quench with water?
Yes—after the carbon–carbon bond-forming steps, a dilute acidic workup (H₃O⁺, NH₄Cl/H₂O, etc.) is the cleanest way to protonate the alkoxide and dissolve Mg salts. Just pre-quench any leftover RMgBr with anhydrous electrophiles so the H₃O⁺ addition is controlled.
Interactive Toolbox
Use these tools to double-check your setup:
- Mechanism Solver — replay the five RDKit frames for “RMgBr + H₃O⁺” on any acid chloride.
- Reaction Solver — plug in your substrate, choose the RMgBr button, and confirm the tertiary alcohol outcome (with warnings when you use <2 eq).
- IUPAC Namer — generate systematic names for both reactants and tertiary alcohol products.
Related Reading
- Acid Chloride → Ketone with Gilman Reagents — when you need to stop after one carbon–carbon bond formation.
- Acid Chloride → Amide with Amines — contrasts nucleophilic acyl substitution with nitrogen nucleophiles.
- Acid Chloride → Primary Alcohol with LiAlH₄ — the hydride route when carbon–carbon bond formation is not required.