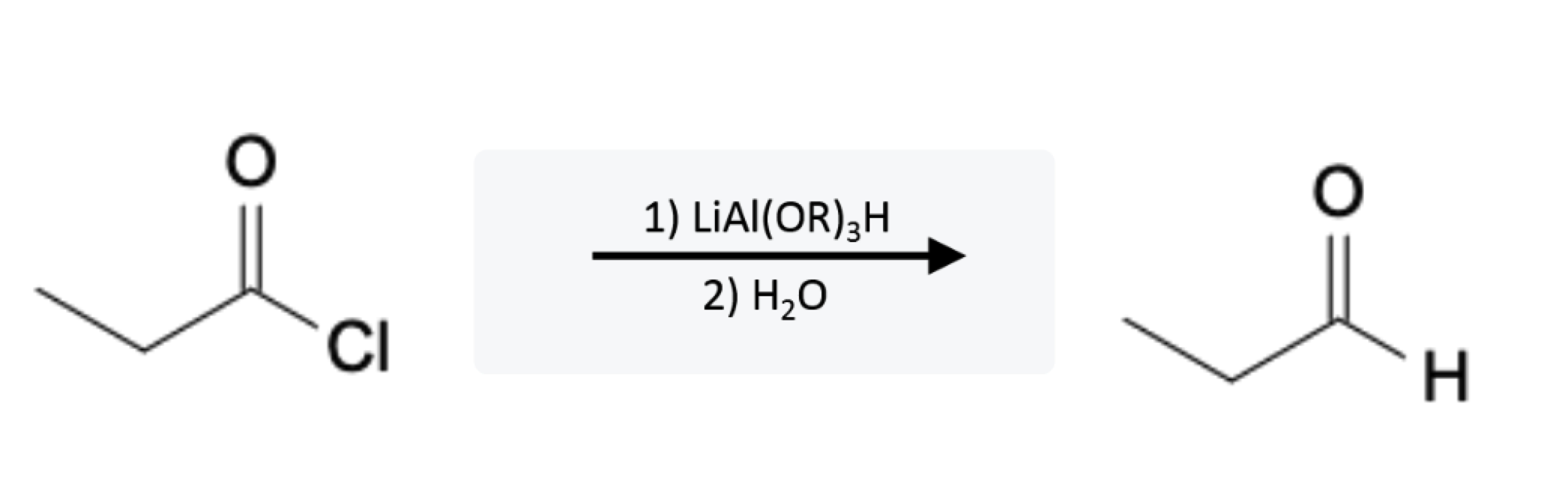
Acid Chloride Reactions: Aldehyde formation using Bulky Aluminum Reducing Agents
Acid chlorides react with bulky aluminum reducing agents, such as lithium tri(tert-butoxy)aluminum hydride (LiAlH(Ot-Bu)3), to form aldehydes:
The reaction mechanism is depicted below:
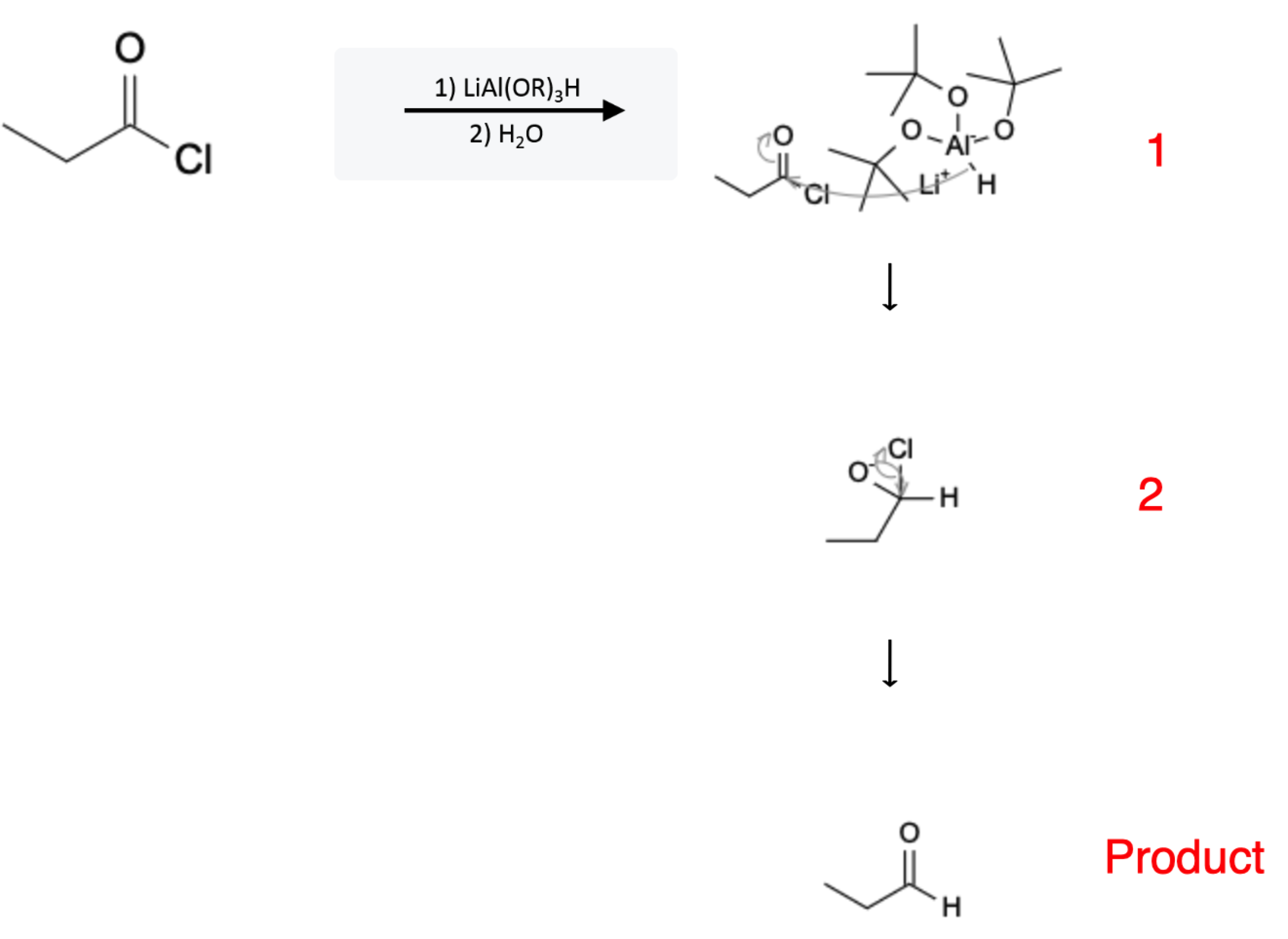
In the first step, the hydride (H-) leaves the LiAl(OtBu)H molecule and attacks the carbonyl carbon, sending electrons from the double bond to the oxygen atom.
In the second step, the electronegative oxygen reestablishes its double bond with the carbon atom, kicking the chloride ion off.
Practice this reaction using our Reaction Solver!