Acid Chloride Reactions: Carboxylic Acid formation Using Water
Acid chlorides react rapidly with water to give carboxylic acids via nucleophilic acyl substitution. Pyridine neutralizes the HCl byproduct (forming pyridinium chloride) and serves as the base in the final deprotonation. The process is closed-shell, fast, and exothermic; the single teaching pathway here is direct water attack.
Teaching pivots: Show addition → tetrahedral intermediate → collapse, with Cl⁻ leaving. Pyridine is both the HCl trap and an optional nucleophilic catalyst (acyl‑pyridinium). Alcohols/amines will outcompete water—exclude them when you want the acid.
Quick Summary
| Reagents/Conditions | Outcome | Notes |
|---|---|---|
| H₂O (often excess) + pyridine (1–2 eq), 0 °C → rt; vigorous stirring. | Acid chloride → carboxylic acid; HCl captured as pyridinium chloride. | Fast, exothermic; keep cold and exclude ROH/RNH₂ if you want the acid. |
Mechanism — Direct H₂O Path (4 Steps)
Class: Nucleophilic acyl substitution; closed-shell.
- H₂O attack (addition): Lone pair on water attacks the acyl carbon; C=O π shifts to O to give a tetrahedral intermediate.
- Collapse (Cl⁻ leaves): The tetrahedral O⁻ re-forms C=O, expelling Cl⁻ to give a protonated carboxylic acid.
- Deprotonation / HCl capture: Pyridine (or water) removes the extra proton; Py + HCl → Py·HCl.
- Product: Neutral carboxylic acid shown; Py·HCl is formed in solution.
Mechanistic Checklist (Exam Focus)
- Show nucleophilic addition → tetrahedral intermediate → collapse with Cl⁻ leaving.
- Depict pyridine capturing HCl (Py·HCl); it can also be shown deprotonating the product.
- No radicals or rearrangements; strictly polar, closed-shell.
- Competing nucleophiles change the product class (ROH → ester, RNH₂ → amide).
Worked Examples
Reactant
Benzoyl chloride
Reagent button
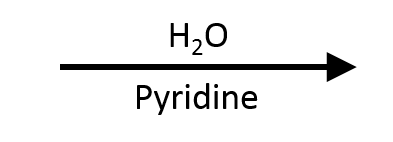
H₂O with pyridine (HCl trap)
Product
Benzoic acid (seafoam OH)
Reactant
Acetyl chloride
Reagent button

H₂O with pyridine
Product
Acetic acid (seafoam OH)
Reactant
Pivaloyl chloride
Reagent button

H₂O with pyridine
Product
Pivalic acid (seafoam OH)
Scope & Limitations
- Works well: Aliphatic and aromatic acid chlorides; aqueous or biphasic setups. Extremely fast and exothermic.
- Functional groups: Acid-sensitive groups may suffer unless buffered; pyridine moderates acidity.
- Selectivity: Exclude ROH/RNH₂ if you want the acid—otherwise ester/amide forms. Sub-stoichiometric water can allow transient anhydride formation (acid chloride + carboxylate).
- Safety: Acid chlorides are lachrymators; pyridine is toxic/odorous—use a hood and add slowly.
Practical Tips
- Add the acid chloride slowly to cold H₂O/pyridine to manage heat and HCl fumes.
- Use ~1–2 eq pyridine (or pyridine solvent) plus excess water to suppress anhydride formation.
- Wash out pyridinium chloride during workup; acidify if an intermediate carboxylate formed.
- If alcohols/amines are present, expect ester/amide instead of acid.
FAQ
Do I need pyridine, or will water alone work?
Water alone hydrolyzes acid chlorides, but pyridine (or bicarbonate/carbonate) is typically used to trap HCl as a salt and keep the medium less acidic.
Why isn’t the acyl‑pyridinium route shown?
We focus on the direct water-addition pathway; pyridine’s main job here is HCl scavenging and final deprotonation.
Can I run this in the presence of alcohols or amines?
They will outcompete water and give esters or amides. Exclude them if you want the acid.
Exam-Style Summary
Acid chloride + H₂O (± pyridine) → carboxylic acid via nucleophilic acyl substitution. Show either direct water attack or an acyl‑pyridinium branch; in both cases pyridine traps HCl as Py·HCl.
Related Reading
- Acid chlorides → primary alcohols (LiAlH₄)
- Acid chlorides → amides with amines
- Acid chlorides → esters with ROH/pyridine
Interactive Toolbox
- Mechanism Solver — toggle direct vs acyl‑pyridinium routes; watch Py·HCl formation with the H₂O/Pyr button.
- Reaction Solver — compare acid chloride outcomes with H₂O vs ROH vs RNH₂.
- IUPAC Namer — verify names for the carboxylic acid products (e.g., benzoic acid, acetic acid).