Acid Chloride Reactions: Ketone formation using Gilmans Reagents
Lithium diorganocuprates (Gilman reagents, R₂CuLi) acylate acid chlorides (RCOCl) to ketones (RCO–R′). The soft cuprate donates one R group; the second stays on copper, so the reaction stops at the ketone rather than over-adding like Grignard or organolithium reagents. Cold, anhydrous ether/THF keeps the cuprate intact; a neutral wash removes copper/lithium salts after collapse.
Highlights: single R transfer (no over-addition), tetrahedral “acylcuprate” intermediate, chloride expulsion, mild workup that simply strips Cu/Li salts.
Quick Summary
Reagents/conditions: R₂CuLi (1.0–1.5 equiv), acid chloride, Et₂O/THF, −78 → 0 °C, inert; neutral NH₄Cl(aq) or dilute acid workup.Outcome: RCOCl → RCO–R′ (ketone). One R transfers; byproducts RCu + LiCl.
Mechanism: Closed-shell acyl substitution: R–Cu attack → tetrahedral “acylcuprate” → collapse, Cl⁻ leaves → ketone; mild workup removes salts.
Selectivity: Softer than RMgX/RLi; ketone survives because the second R does not add.
Exam hook: Contrast with Grignard/organolithium, which over-add to give alcohols.
Mechanism — 3 Steps
Mechanistic Checklist
- One R transfer only; the second R stays on copper (RCu byproduct).
- Chloride leaves with Cu/Li salts; no protonation of carbonyl needed.
- Run cold and anhydrous; RMgX/RLi contamination can over-add to the ketone.
- Ketones formed are stable to further cuprate addition under these conditions.
Worked Examples
Reactant
Reagent
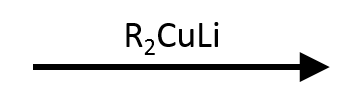
Me₂CuLi; one Me transfers, one stays on Cu.
Product
Acetophenone (IUPAC: 1-phenylethan-1-one).
Reactant
Reagent
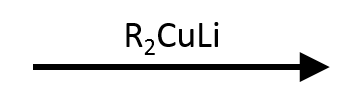
Ph₂CuLi; transfers one phenyl.
Product
tert-Butyl phenyl ketone (IUPAC: 2-methylpropan-2-yl phenyl ketone).
Reactant
Reagent
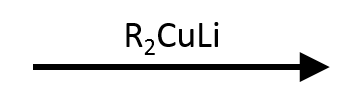
n-Bu₂CuLi; transfers one n-Bu.
Product
2-Hexanone (IUPAC: hexan-2-one).
Scope & Limitations
- Good: Aliphatic, aromatic, and vinyl acid chlorides; symmetric dialkyl or diaryl cuprates; vinyl cuprates.
- Less suitable: Esters, amides, thioesters (too sluggish for cuprates without activation).
- Compatibility: Halides, nitriles, ethers often survive; avoid protic solvents that quench the cuprate.
- Risk: Contaminating RMgX/RLi will over-add to ketones—prepare cuprate cleanly.
Practical Tips
- Form R₂CuLi from RLi + CuI at −78 °C; allow complete transmetalation before adding acid chloride.
- Add acid chloride slowly at −78 → 0 °C to maintain selectivity and avoid over-addition.
- Quench with cold NH₄Cl(aq) or dilute acid; remove Cu/Li salts by filtration/extraction.
Exam-Style Summary
Acid chloride + R₂CuLi → tetrahedral acylcuprate → collapse (Cl⁻ leaves with Cu/Li) → ketone. Only one R transfers; ketone is stable to further cuprate under standard conditions. Cold, anhydrous ether/THF required.
FAQ
Does R₂CuLi over-add to acid chlorides? No—only one R transfers; the second remains on copper.
Why use cold conditions? Keeps the cuprate intact and suppresses side reactions or over-addition by stray RMgX/RLi.
Can cuprates acylate esters? Esters are generally too unreactive toward cuprates; acid chlorides are the preferred electrophile.
Interactive Toolbox
- Mechanism Solver — animate R–Cu attack → collapse → workup; toggle R₂CuLi vs RMgX for contrast.
- Reaction Solver — compare acid chloride outcomes under Gilman vs Grignard conditions.
- IUPAC Namer — generate names like acetophenone or tert-butyl phenyl ketone.