Acid Chloride Reactions: Reduction of Acid Chlorides using LiAlH4 to form Alcohols
Lithium aluminum hydride (LiAlH₄) reduces acid chlorides (RCOCl) rapidly and completely to primary alcohols. Two hydride equivalents add across the acyl unit: the first creates a tetrahedral intermediate that collapses to an aldehyde, the second immediately reduces that aldehyde to an alkoxide. Aqueous workup (H₃O⁺) protonates the alkoxide to give the neutral alcohol.
Teaching pivots: Emphasize two hydride additions (acid chloride → aldehyde → alkoxide → alcohol), chloride expulsion, and careful staged quench. LiAlH₄ will not stop at the aldehyde; use selective hydrides if that’s the goal.
Quick Summary
| Reagents/Conditions | Outcome | Notes |
|---|---|---|
| LiAlH₄ (Et₂O/THF, strictly anhydrous, 0 °C → rt), then H₃O⁺ quench | RCOCl → RCH₂OH (primary alcohol) | Two hydride transfers; aldehyde not isolable; violent with water—quench cold and slowly. |
Mechanism — 5 Steps (Closed-Shell Nucleophilic Acyl Substitution)
- Hydride addition to the acyl carbon: AlH₄⁻ delivers H⁻ (explicit H–Al bond) to the carbonyl carbon; π electrons shift to oxygen → tetrahedral alkoxide with chloride still attached.
Mechanistic Checklist (Exam Focus)
- Draw two hydride additions: acid chloride → aldehyde (in situ) → alkoxide → alcohol (workup).
- Show a tetrahedral intermediate and chloride expulsion; no radicals or rearrangements.
- LiAlH₄ does not stop at the aldehyde; use LiAlH(O‑tBu)₃ or Rosenmund if you need one.
- Acid or water before completion quenches LiAlH₄ and stalls the reduction.
- No stereocenter survives at the acyl carbon—product carbon becomes CH₂.
Worked Examples
Reactant
Reagent button
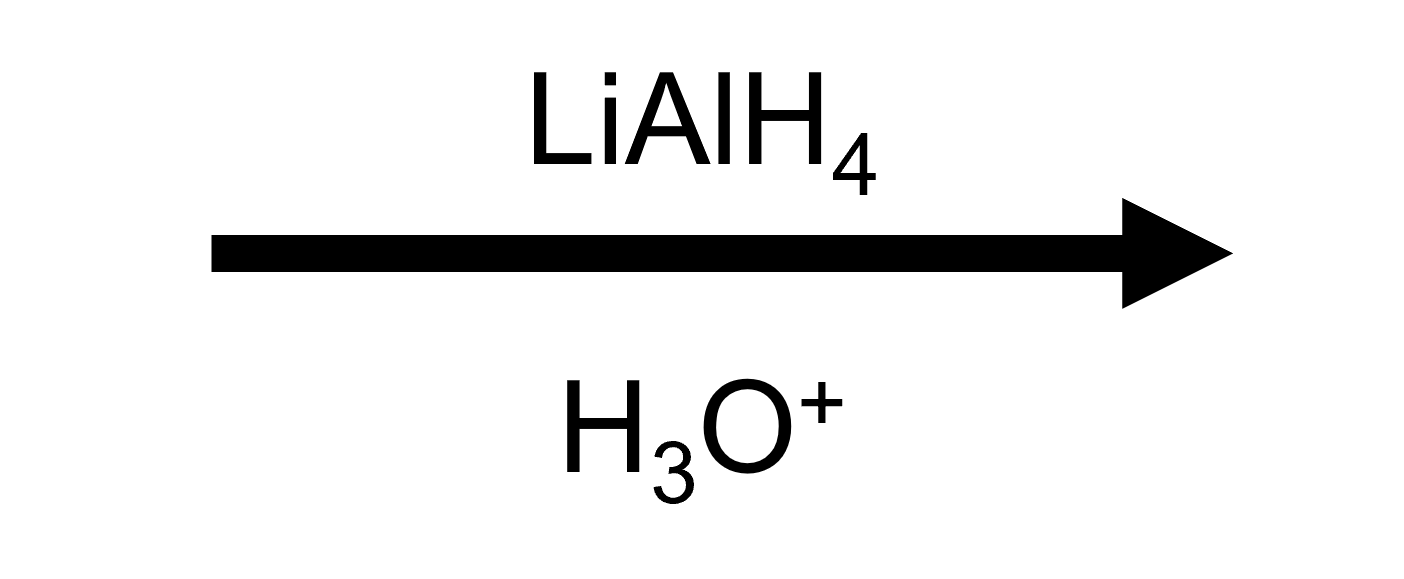
LiAlH₄, then H₃O⁺
Product
Benzyl alcohol (seafoam OH highlight)
Reactant
Reagent button
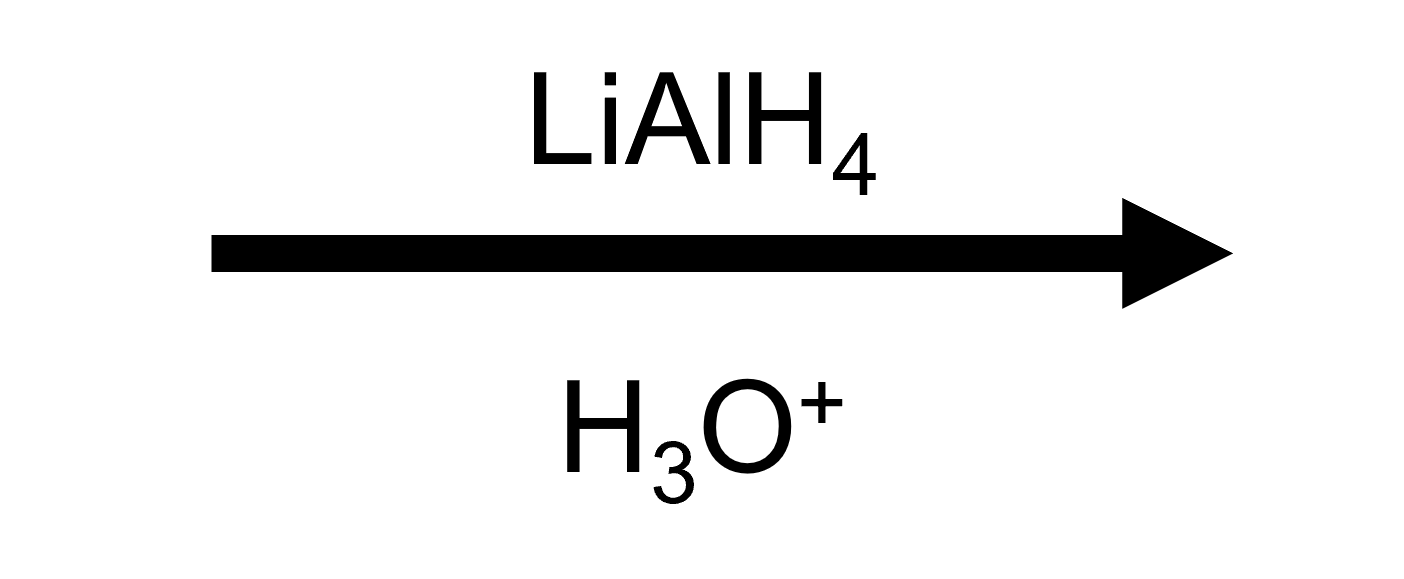
LiAlH₄, then H₃O⁺
Product
1-octanol (seafoam OH highlight)
Reactant
Reagent button
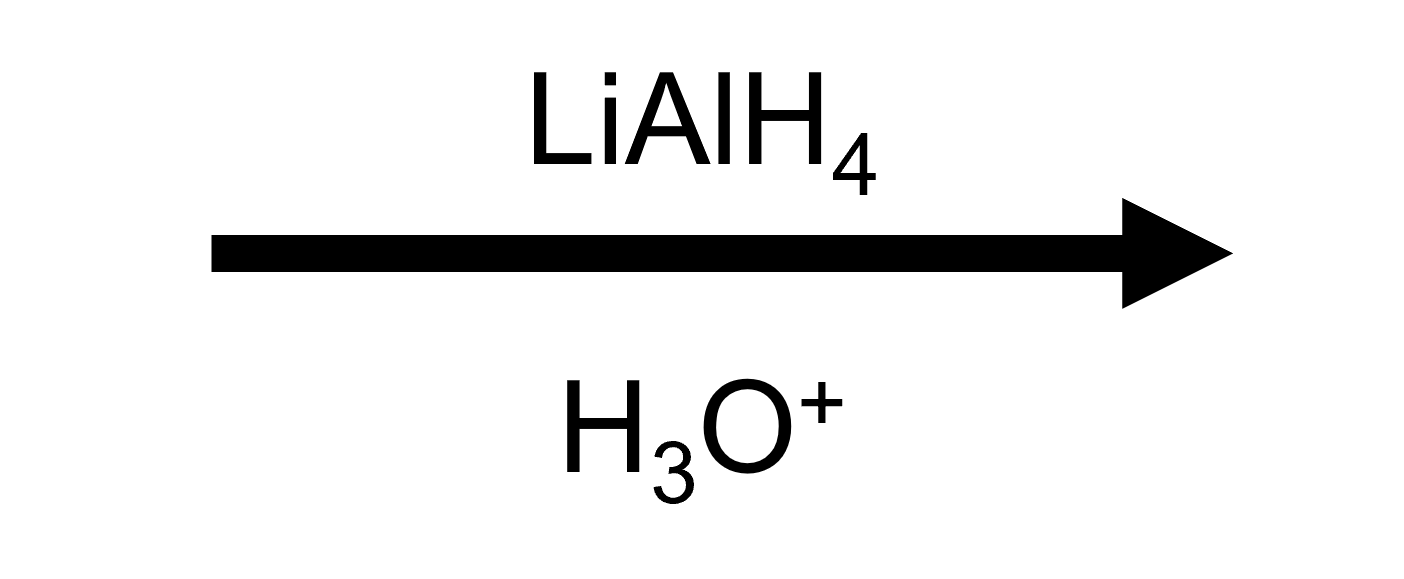
LiAlH₄, then H₃O⁺
Product
p-Methoxybenzyl alcohol (seafoam OH highlight)
Scope & Limitations
- Works: Aliphatic and aromatic acid chlorides; rapid and high-yielding under dry ether/THF.
- Chemoselectivity: LiAlH₄ also reduces esters and anhydrides to alcohols and amides/nitriles to amines—avoid sensitive groups or protect them.
- Aldehyde access: Choose LiAlH(O‑tBu)₃ at low temperature or Rosenmund hydrogenation; LiAlH₄ is too strong to stop early.
- Stoichiometry: ≥2 hydride equivalents per acyl group; practical excess is common.
- Safety: Pyrophoric; violent with water. Quench cold, slowly, and in stages.
Practical Tips (Lab)
- Dry glassware and inert atmosphere; charge LiAlH₄ first, then add the acid chloride solution slowly at 0 °C.
- Control exotherm and gas evolution; never add LiAlH₄ to water.
- Stage the quench: cautious water addition → mild base (e.g., NaOH) to break Al complexes → full acidic workup (H₃O⁺) to release the alcohol.
- Use ≥2 eq hydride per acyl unit; more if other reducible groups are present.
Exam-Style Summary
RCOCl —(LiAlH₄, dry ether/THF)→ [tetrahedral] → aldehyde (in situ) —(LiAlH₄)→ RCH₂O⁻ —(H₃O⁺)→ RCH₂OH. Two hydride transfers; chloride leaves in the collapse; aldehyde is not isolated.
FAQ
Does LiAlH₄ stop at an aldehyde?
No. It reduces acid chlorides past the aldehyde to the primary alcohol. Use LiAlH(O‑tBu)₃ or Rosenmund to isolate an aldehyde.
How many hydride equivalents are needed?
At least two per acyl group; use excess to account for side consumption.
Which solvents are typical?
Dry diethyl ether or THF under N₂/Ar.
How should I quench LiAlH₄ safely?
Cold, dropwise water, then mild base to dismantle Al complexes, then acidic workup (H₃O⁺).
Interactive Toolbox
- Mechanism Solver — animate both hydride additions, chloride expulsion, and the acidic workup.
- Reaction Solver — predict primary alcohols from acid chlorides; flag co-present esters/amides as over-reduction risks.
- IUPAC Namer — caption products such as benzyl alcohol or 1-octanol without exposing SMILES.
Related Reading
- Ester → alcohol (LiAlH₄, then H₃O⁺) — compare over-reduction and workup details across acyl substrates.
- Aldehyde/ketone → alcohol (LiAlH₄) — closed-shell hydride transfer for simpler carbonyls.
- Acid chloride hydrolysis (H₂O, pyridine) — contrast reduction vs hydrolysis outcomes.