Alcohol Dehydration to Alkenes with Strong Bronsted Acids (H2SO4, H3PO4, p-TsOH, MsOH) — Unified E1/E2-like Mechanism
Alcohol Reactions: Acid-Catalyzed Dehydration to Alkenes (H2SO4, H3PO4, p-TsOH, MsOH)
Concentrated strong Bronsted acids convert alcohols to alkenes by eliminating water. Secondary and tertiary alcohols prefer an E1 sequence: protonate, lose water to a carbocation, then deprotonate to the most substituted (often Zaitsev) alkene. Primary alcohols avoid free primary carbocations, instead eliminating in one concerted, E2-like step from the protonated alcohol (ROH2+) so rearrangements do not occur. The four acids highlighted here share identical oxygen-centered chemistry-the acid primarily influences handling, oxidation risk, and how you badge the mechanism.
- H2SO4 (concentrated): very strong and oxidizing; classical textbook reagent.
- H3PO4 (concentrated): strong but non-oxidizing; gentler when substrates scorch with sulfuric acid.
- p-TsOH (p-toluenesulfonic acid): bench-stable solid, non-oxidizing, often with Dean-Stark setups in toluene.
- MsOH (methanesulfonic acid): strong, non-oxidizing liquid; convenient alternative for solution processes.
The unified mechanism below renders different acid labels and conjugate-base tags while keeping all curved arrows anchored to the same alcohol oxygen, just as the interactive builder expects.
Introduction
Concentrated strong Bronsted acids protonate alcohols, turning a poor hydroxyl leaving group into water and unlocking alkene formation. Secondary and tertiary substrates form carbocations after water departure, so Zaitsev alkenes and rearrangements (hydride or alkyl shifts, ring expansions) are on the table. Primary alcohols instead eject water at the same time the beta proton is removed; no discrete carbocation means no rearrangement. The acids showcased-H2SO4, H3PO4, p-TsOH, and MsOH-differ in oxidation risk, physical form, and handling, but the mechanistic arrows never move away from the alcohol oxygen. Heat or continuous water removal drives the equilibrium to alkene over ether formation.
Quick Summary
- Reagents and conditions: concentrated H2SO4, concentrated H3PO4, p-TsOH, or MsOH; heat and/or water removal push equilibrium to alkene.
- Branching logic: secondary, tertiary, benzylic, and allylic substrates follow E1 (carbocation); primary substrates follow an E2-like concerted loss from ROH2+.
- Selectivity: favors Zaitsev substitution, with conjugated and trans alkenes winning when available; Hofmann only if enforced by sterics or poor beta-H access.
- Rearrangements: only relevant on the E1 branch where carbocations can shift.
- Competing reactions: at lower temperature in alcohol solvent, intermolecular ether formation can rival dehydration (especially primary substrates).
Mechanism (unified frames)
Shared activation (all paths)
Path A – Secondary/Tertiary (E1; rearrangement enabled)
Ionization (rate determining)
Optional rearrangement
β-Deprotonation → alkene
Product frame
Path B – Primary (concerted E2-like)
Concerted β-deprotonation + water loss
Product frame
Mechanistic Checklist
- Choose the path by substrate class: secondary, tertiary, benzylic, and allylic run through E1; primary runs through the E2-like frame.
- Rearrangements are only possible on the E1 branch and should be gated by
allowRearrangementin tooling. - Regiochemistry defaults to Zaitsev; conjugation can outrank simple substitution counts.
- Stereochemistry: when defined, trans alkenes dominate; note conjugated vs isolated double bonds.
- Competing reactions: at low temperature in alcohol solvent, flag possible ether formation; polymerization can appear with activated alkenes under harsh acid.
- Contrast: POCl3/pyridine executes E2 dehydration without carbocations—call this out when comparing study aids.
Worked Examples
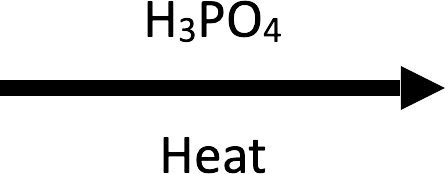
Example 1 – 3-methylbutan-2-ol → 2-methylbut-2-ene (H3PO4/Δ)
Hydride migration (Step 3) moves the positive charge to the tertiary centre before β-deprotonation furnishes the Zaitsev alkene.
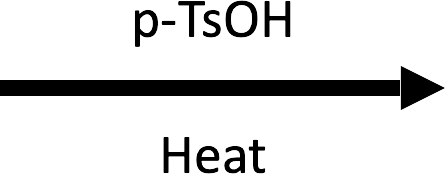
Example 2 – 1-methylcyclobutan-1-ol → 1-methylcyclopentene (p-TsOH/Δ)
Ring expansion in the optional rearrangement frame relieves four-membered strain before β-elimination gives the more substituted cyclopentene.
Example 3 – Butan-1-ol → (Z)-but-2-ene (major) (MsOH/Δ)
Primary substrates follow the concerted E2-like path; the conjugate base removes the syn β-H while water departs, giving predominantly (Z)-but-2-ene.

Example 4 – 1-phenylethan-1-ol → Styrene (H2SO4/Δ)
The benzylic carbocation is already resonance-stabilised, so rearrangement is unnecessary before β-deprotonation forms styrene.
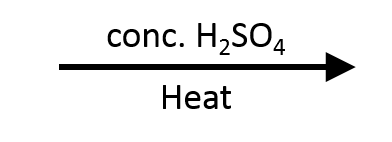
Edge Cases & Exam Traps
- Primary substrates should never display a free primary carbocation—use the E2-like frame.
- Always check for adjacent tertiary centers or ring expansion opportunities when you choose the E1 branch.
- Conjugated alkene options often dominate even if they are not the most substituted.
- Low temperature and alcohol solvent raise the odds of ether formation; call out the competition in problem-solving tools.
- Sulfuric acid can char sensitive substrates; remind learners about the gentler alternatives (H3PO4, p-TsOH, MsOH).
Practical Tips
- Heat gradually or remove water to drive equilibrium (Dean-Stark for p-TsOH, azeotropic distillation, or sparging).
- Choose acid by sensitivity: H3PO4, p-TsOH, and MsOH are non-oxidizing alternatives when H2SO4 is too harsh.
- Watch for polymerization with very reactive alkenes (isobutylene analogs) under strong acid.
- Always apply appropriate PPE and neutralization protocols for concentrated acids at elevated temperatures.
Exam-Style Summary
Protonate the alcohol -> ROH2+. Secondary/tertiary/benzylic/allylic substrates: water leaves (rate determining) -> carbocation -> beta deprotonation -> alkene (Zaitsev, trans/conjugated favored, rearrangements possible). Primary substrates: concerted beta deprotonation as water departs from ROH2+ -> alkene (no rearrangement). Four acids behave identically at the electron-flow level-only the overlay badge and conjugate-base tag change.
Related Reading
- Alcohol → alkyl bromide (PBr₃)
- Alcohol → alkyl chloride (SOCl₂)
- Appel reaction: alcohol → alkyl halide
Interactive Toolbox
- Mechanism Builder: Mechanism Solver, pick your alcohol, then toggle the acids to see the mechanism.
- Reaction Solver: Reaction Solver with Zaitsev/conjugation weighting and water-removal flags to visualize products.
- IUPAC Namer: IUPAC Namer (styrene, 2-methylbut-2-ene, etc.) directly from drawn structures.