TsCl/Pyridine: Alcohol to Tosylate (ROTs) + What Happens Next (SN2 vs E2)
Exam Answer
- Reagents
- TsCl, pyridine
- Major outcome
- ROH to ROTs (activation)
- Selectivity
- Tosylation is not substitution at carbon; follow-up controls outcome
Common traps
- ROTs formation does not invert carbon stereochemistry
- 3 degree substrates: no SN2 follow-up; elimination/solvolysis depends on conditions
- Do not claim NaCl gives RCl unless explicitly SN1/ionizing solvent conditions
ROTs Follow-Up Chooser (SN2 vs E2 vs Solvolysis)
Jump to full playbook| Substrate plus follow-up | Pathway | Notes |
|---|---|---|
| 1 degree ROTs plus strong nucleophile | SN2 | inversion |
| 2 degree ROTs plus strong nucleophile (aprotic) | SN2 competes | some elimination possible |
| 2 degree ROTs plus strong base / heat | E2 | alkene |
| 3 degree ROTs | E1/E2/solvolysis | depends on conditions |
TsCl/pyridine converts an alcohol (ROH) into a tosylate (ROTs), a much better leaving group. Configuration at the carbon bearing oxygen is retained during tosylation; the follow-up reagent dictates the functional group. Pair ROTs with halides, azide/cyanide, acetate, or small alkoxides for SN2 substitution (inversion). Reach for bulky t-BuO⁻/t-BuONa, non-nucleophilic DBU/DBN, or LiAlH₄ to steer elimination or reduction, and reserve warm ROH/H₂O for solvolysis concept panels.
Exam Answer
Reagents: TsCl, pyridine.
Major outcome: ROH → ROTs (activation; carbon skeleton unchanged).
Selectivity: Tosylation retains configuration at carbon; SN2 follow-up on ROTs inverts.
Trap: TsCl does not give RCl directly—ROTs is the checkpoint.
Deciding between SN2 and E2? Jump to the ROTs follow-up chooser below.
Quick FAQs (TsCl/pyridine)
Does TsCl invert stereochemistry? Tosylation retains configuration; inversion only happens in an SN2 follow-up step on ROTs.
Why pyridine? It scavenges HCl and drives tosylate formation without creating a carbocation, keeping the C–O stereochemistry intact.
Can tertiary alcohols “convert” cleanly? Tosylates can form, but SN2 is blocked; outcomes are elimination or SN1/E1 solvolysis depending on conditions.
Quick Summary
- Activation: TsCl/pyridine converts ROH to ROTs in two steps (sulfur attack, deprotonation) with complete retention at the carbon center.
- Downstream control: The second reagent defines the functional group: halides, azide/cyanide, acetate, alkoxides, hydride, bulky bases (t-BuO⁻/t-BuONa), and non-nucleophilic bases (DBU/DBN) all start from the same ROTs checkpoint.
- Substrate-class trends: Primary ROTs race through SN2; secondary sites juggle SN2 vs E2; tertiary sites eliminate (DBU/DBN or t-BuO⁻) rather than substitute.
- Stereochemistry accounting: Tosylation (retention) × SN2 (inversion) = net inversion; pure E2 retains the β-anti relationship and gives alkenes set by anti-periplanar access.
- Solvent/base tuning: Polar aprotic solvents accelerate halide or azide SN2; matching alcohol/base pairs help NaOEt; bulky or non-nucleophilic bases need heat and enforce elimination.
Mechanism — TsCl Activation, Then Pick Your Follow-Up
The frames below come directly from the OrgoSolver Mechanism Solver using 2-butanol as the substrate. Open the solver and select the TsCl/pyridine option, then explore the variants shown to see the same electron-pushing views.
Shared activation — TsCl/pyridine upgrades ROH → ROTs
Branch A — Halide SN2 (NaBr) delivers inversion
Branch B — Non-nucleophilic base E2 (DBU) targets the anti β-H
Why the follow-up reagent matters
Tosylation merely upgrades the leaving group. Substitution, elimination, reduction, or solvolysis only occur after you add the second reagent. The mechanism suite routes every preset through the same TsCl activation; downstream logic (NaX, NaN₃, NaOEt, t-BuOK, DBU, DBN, LiAlH₄, NaOAc, solvolysis, etc.) determines the functional group and stereochemical fate.
ROTs Follow-Up Chooser (SN2 vs E2 vs Solvolysis)
This table is the exam decision step: pick follow-up by (1) substrate class, (2) nucleophile vs base strength, (3) temperature, (4) anti β-H geometry.
| Reagents & group |
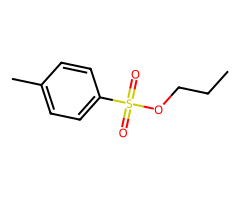 Primary ROTs
Primary ROTs
|
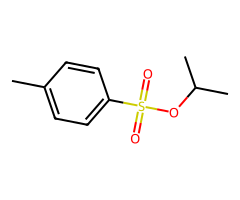 Secondary ROTs
Secondary ROTs
|
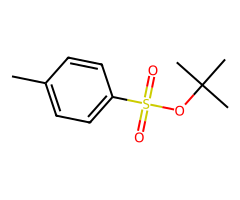 Tertiary ROTs
Tertiary ROTs
|
Highlights |
|---|---|---|---|---|
| Halides | 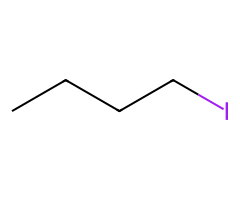 Rapid SN2 → inversion (acetone/DMF). | 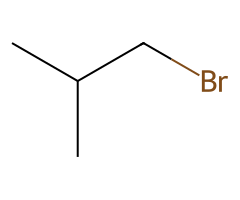 SN2 at cool temps; heat or steric bulk → some E2. | ![Tertiary ROTs: SN1/E1 solvolysis or elimination; chloride capture only if ionizing solvent, heat, and high [Cl⁻].](/assets/reaction-library/alcohol-tscl-halide-tertiary-product.png) No SN2; conditions decide SN1/E1 vs elimination. | Finkelstein (I⁻) rescues sluggish cases. |
| Pseudohalides | 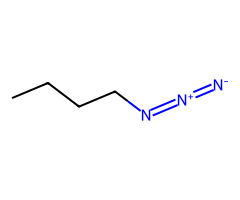 Extremely fast SN2 → inversion. | 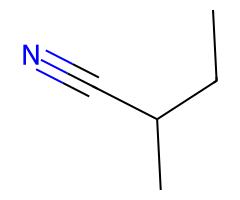 SN2 favored when cool/aprotic; warming invites E2. |  For tertiary ROTs, pick elimination (strong base) or SN1/E1 solvolysis in ionizing solvent; CN⁻ is not the path. | Allylic SN2′ common; emphasize azide/cyanide safety. |
| Small alkoxides | 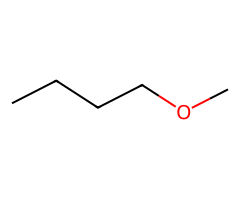 SN2 → ether (inversion). | 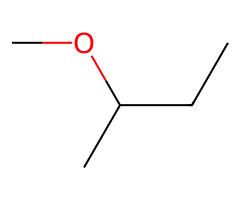 SN2/E2 tug-of-war; temperature decides. | 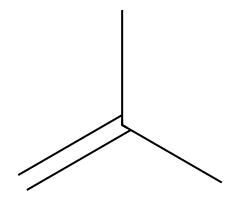 E2 only. | Classic Williamson ether synthesis from ROTs. |
| Bulky alkoxides | 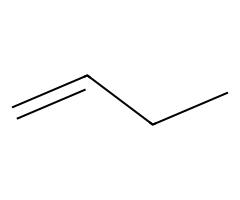 Slower SN2; E2 gives the terminal alkene here. | 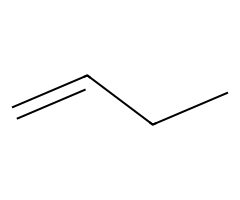 Hofmann-leaning E2 dominates. | 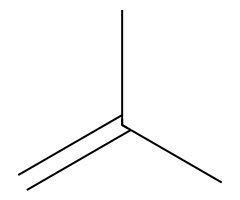 E2 exclusively. | Requires anti β-H; chairs must flip axial. |
| Non-nucleophilic bases | 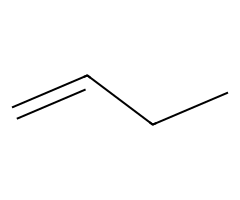 Slow E2; primary substrates give the terminal alkene available. | 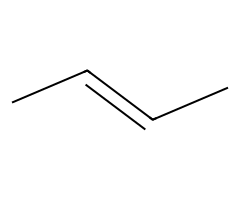 Often Zaitsev E2, but substrate geometry/anti β-H access controls outcome. |  E2 exclusively. | DBU/DBN abstract β-H without competing SN2. |
Mechanistic Checklist
- Two-step activation (attack + deprotonation) gives ROTs with complete retention. All stereochemical changes happen later.
- Pick the follow-up by substrate class: primary favors halide/azide/acetate SN2; secondary needs condition control; tertiary demands elimination (t-BuO⁻, DBU, DBN).
- Non-nucleophilic bases (DBU/DBN) are often Zaitsev-leaning eliminators but still require anti-periplanar β-H alignment.
- Bulky t-BuO⁻/t-BuONa bias Hofmann when anti β-H access to the more substituted site is blocked.
- Neopentyl and highly hindered secondary centers remain slow for SN2 even with ROTs; plan for elimination or activation tweaks.
- Benzylic/allylic substrates accelerate SN2 and enable SN2′ mixtures; be ready to discuss regiochemical outcomes.
- Safety: azide (energetic), cyanide (toxic), and LiAlH₄ (vigorous quenches) require dedicated notes in lab writeups.
Worked Examples
Each row highlights how the same TsCl activation pivots once you change the follow-up reagent. The reagent badges align with options inside the Mechanism Solver; load the TsCl/pyridine option and compare the substrate and product snapshots.
| Alcohol class & substrate | Reagents in play | Representative product |
|---|---|---|
|
Halide presets (acetone or DMF, cool) — SN2 inversion of the activated primary center. |

|
|
Small alkoxides — Williamson ether formation dominates when conditions stay cold and aprotic. |

|
|
Non-nucleophilic or bulky bases drive anti-periplanar E2 (check trans-diaxial β-H availability). |
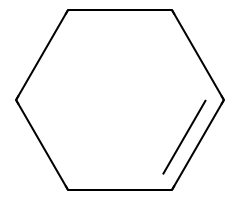
|
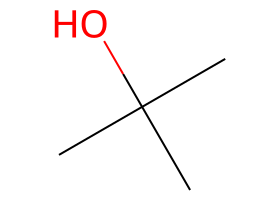
|
Tertiary ROTs eliminate — bulky bases bias Hofmann; DBU/DBN often give the more substituted alkene when anti β-H is available. |

|
Edge Cases & Exam Traps
- Neopentyl SN2 stays painfully slow—call out when problems try to trick students with “primary” labeling.
- Cyclic substrates need ROTs axial for E2. If no anti β-H is available, elimination stalls even with DBU.
- Excess heat pushes secondary ROTs toward elimination even with “nucleophilic” reagents (NaOEt, NaCN).
- Benzylic/allylic ROTs may undergo SN1 under ionizing conditions; emphasize the competition when the question expects stereochemical mixtures.
- LiAlH₄ reduces primary/secondary ROTs; tertiary tosylates do not reduce by SN2—expect elimination or failure instead.
- Free amines react with TsCl before alcohols; protect or reverse the order when both functional groups are present.
- Reminder: aryl/vinyl carbons do not perform SN2; mention cross-coupling or other tactics instead.
Practical Tips
- Maintain dry conditions (pyridine or Et₃N) during tosylation; quench carefully before swapping solvents.
- After activation, switch to solvent systems that match the reagent: acetone/DMF for halides or azide/cyanide, t-BuOH for t-BuO⁻/t-BuONa, MeCN or toluene for DBU/DBN.
- Track stereochemistry explicitly: ROTs formation (retention) followed by the chosen SN2 (inversion) or E2 (alkene geometry) step.
- For cyclic eliminations, pre-draw the chair to ensure an axial β-H. If absent, choose a different substrate or expect slow reaction.
- NaBr often outpaces NaCl for SN2; Finkelstein (NaI) can rescue stuck primaries.
- Highlight safety for NaN₃ (shock sensitivity), NaCN/KCN (toxicity), and LiAlH₄ (exothermic quench) in lab prep notes.
Exam-Style Summary
Activate with TsCl/pyridine (retention). From the ROTs checkpoint:
- Halides / pseudohalides / acetate / small alkoxides: Primary (fast SN2) > secondary (condition-sensitive) > tertiary (no SN2). Net inversion relative to the starting alcohol.
- Bulky alkoxides (t-BuO⁻/t-BuONa): Hofmann-leaning E2; anti β-H mandatory.
- DBU / DBN: Often Zaitsev E2 on secondary/tertiary ROTs when anti β-H is available; minimal nucleophilicity.
- LiAlH₄: Primary/secondary ROTs → alkanes (SN2); tertiary gives elimination or no useful reduction.
- Solvolysis: Warm ROH/H₂O yields SN1/E1 mixtures; use for conceptual contrast, not synthesis.
Related Reading
- Alcohol → alkyl bromide (PBr₃)
- Alcohol → alkyl chloride (SOCl₂)
- Appel reaction: alcohol → alkyl halide
Interactive Toolbox
- Mechanism Solver — pick TsCl/pyridine, choose substrates of different classes, and step through activation plus the NaBr/DBU (or any other) branches shown above.
- Reaction Solver — compare substitution vs. elimination predictions by toggling base strength, sterics, solvent, and temperature.
- IUPAC Namer — confirm product names (alkyl halides, ethers, alkenes) directly from drawn structures without exposing learners to structural encodings.