Williamson Ether Synthesis (RO- + R-X to R-O-R)
Exam Answer
- Reagents
- 1) base to form RO- (often NaH) 2) primary R-X or R-OTs
- Major outcome
- Ether formation via SN2
- Selectivity
- Requires an unhindered electrophile
Common traps
- 3 degree electrophiles fail SN2 (E2 dominates)
- 2 degree electrophiles compete with E2
- Protic solvents weaken nucleophile and reduce SN2 rate
Choose the electrophile
| Electrophile | Outcome | Notes |
|---|---|---|
| 1 degree R-X / R-OTs | SN2 ether formation | best case |
| 2 degree R-X | SN2 vs E2 | depends on base/solvent/heat |
| 3 degree R-X | E2 | no SN2 |
Sodium hydride (NaH) strips alcohols of their acidic proton, releasing H₂ and creating a highly nucleophilic alkoxide. Once formed, that alkoxide performs a backside SN2 attack on a compatible alkyl halide (R′–X) to build an ether. If the molecule already houses both the alcohol and a pendant halide, the same sequence becomes intramolecular and closes a ring. Secondary/tertiary electrophiles resist substitution—expect E2 elimination instead.
Comparisons: use PBr₃ or SOCl₂ when you need to convert the alcohol itself into a halide before substitution, or Appel conditions for mild halide installation.
Quick Summary
- Reagents: alcohol (R–OH), strong base (NaH preferred), alkyl halide electrophile (R′–Cl/Br/I or sulfonate ester), polar aprotic solvent (THF/DMF/DMSO). Degas the H₂ before adding the halide.
- Scope: methyl and primary halides react fastest; allylic/benzylic primaries are excellent. Secondary halides give mixtures or elimination; tertiary halides are purely E2.
- Sequence: (1) NaH deprotonation → alkoxide + H₂; (2) add R′–X for SN2; (3) intramolecular halides cyclise spontaneously.
- Intramolecular trigger: HO–(CH₂)ₙ–X forms 5–7 membered rings readily; emphasise the pendant “R” replacing X when describing products.
- Competing E2: secondary or tertiary alkyl halides default to elimination; no ether forms (see Worked Example C).
- Variants: phenoxides undergo Williamson ether formation with simple halides to give aryl ethers; sulfonate esters replace halides when a non-halide leaving group is needed.
Mechanism — Step 1: NaH Deprotonation
- Irreversibility: gaseous H₂ leaves the mixture, driving the equilibrium toward alkoxide formation.
- Practical note: wait until gas evolution ceases; residual NaH indicates complete deprotonation.
Mechanism — Step 2: SN2 on R′–X
- Stereochemistry: inversion at the electrophilic carbon (classic SN2).
- Leaving groups: iodides > bromides > chlorides; tosylates/mesylates work equivalently.
- Solvent: polar aprotics keep the alkoxide solvated yet reactive.
Intramolecular Williamson (Ring Closure)
- Outcome: crown ethers, lactones, and mixed rings form rapidly; external electrophiles often cannot compete.
- Design tip: tether length dictates ring size; ensure minimal strain for efficient cyclisation.
Competing E2 with Secondary/Tertiary R′–X
Secondary or tertiary alkyl halides cannot accommodate backside attack. The alkoxide switches to a base, abstracting a β-hydrogen and forming an alkene while X⁻ departs. Worked Example C highlights this failed ether attempt: the secondary halide is consumed via E2, so no ether is produced.
Worked Examples
1. n-Propanol + Methyl Bromide → Methyl n-propyl ether
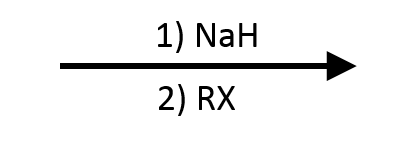
Primary alkoxide + methyl halide → clean SN2 ether formation with inversion at the electrophilic carbon.
2. Phenoxide + Methyl Bromide → Anisole
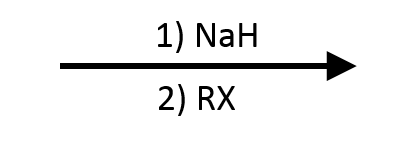
Phenoxide reacts with methyl bromide to give anisole—classic Williamson extension to aryl ethers.
3. Methoxide + sec-Butyl bromide (E2 dominates)
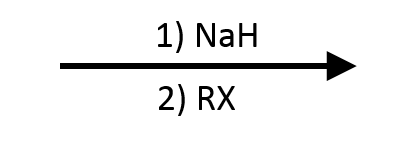 +
+
Deprotonated methanol attacks secondary sec-butyl bromide as a base, not a nucleophile, giving 2-butene via E2. No ether forms—secondary/tertiary electrophiles collapse to elimination.
Mechanistic Checklist
- Generate alkoxide completely (monitor for H₂ bubbles, add halide only after evolution stops).
- Choose a viable electrophile (methyl/primary/allylic/benzylic). Secondary/tertiary → elimination.
- Use polar aprotic solvent to keep the alkoxide reactive (THF, DMF, DMSO).
- Watch for intramolecular halides—they outcompete intermolecular ones.
- Keep water/ROH out once the alkoxide forms; proton donors quench the nucleophile.
- Consider leaving group swaps (tosylate/mesylate) if a halide isn’t available.
- State the R′ fragment explicitly in products/exams so students know which carbon chain moves.
Exam-Style Summary
- Predictive prompt: “NaH, then 1-bromopropane with methanol.” → methoxide SN2 to give methyl propyl ether (inversion at the brominated carbon).
- Intramolecular prompt: “HO–(CH₂)₅–Br + NaH.” → deprotonate and cyclise to THP (five-member ring).
- Failure prompt: “NaH + tert-butyl bromide.” → elimination only; emphasise E2 rationale.
- Mechanism request: always draw Step 1 (deprotonation) before Step 2 (SN2 or E2) to highlight sequencing.
Interactive Toolbox
- Mechanism Solver – Draw the alcohol, pick “NaH / RX,” and preview the RDKit-rendered SN2 and intramolecular pathways directly from RDKit.
- Reaction Solver – Search “NaH” or “Williamson” to quiz yourself on reagents, keywords, and short-form prompts.
- IUPAC Namer – Double-check ether product names once you assign the R and R′ chains.
Ready to practice? Launch the Mechanism Solver or Reaction Solver and run the Williamson ether synthesis with your own substrates.