Aldehyde → Carboxylic Acid with Chromate (Cr(VI))
Aldehyde Oxidation to Carboxylic Acids with Chromate (Cr(VI))
Under aqueous acidic conditions, Cr(VI) oxidants—Na₂Cr₂O₇/H₂SO₄, K₂Cr₂O₇/H₂SO₄, CrO₃/H₂SO₄ (Jones reagent), or preformed H₂CrO₄—rapidly convert aldehydes into carboxylic acids. In water an aldehyde hydrates to its gem-diol; one hydroxyl esterifies with chromic acid to give a chromate ester, and β-elimination (formally hydride transfer to Cr(VI)) generates the carboxylic acid while chromium is reduced to Cr(III). The four reagent buttons shown in the UI all converge on this shared mechanism; only the oxidant label/solvent overlay changes.
Quick Summary
- Reagents/conditions: Na₂Cr₂O₇ or K₂Cr₂O₇ with H₂SO₄ (aq), CrO₃/H₂SO₄ in acetone–water (Jones), or H₂CrO₄ (aq); 0–25 °C with water present.
- Outcome: Aldehyde → carboxylic acid (formaldehyde → formic acid; longer chains → corresponding acids).
- Mechanistic spine: Hydrate formation → chromate ester → β-elimination / hydride transfer → carboxylic acid (Cr(VI) → Cr(III)).
- Selectivity: Ketones do not over-oxidise to acids here; this page focuses on aldehydes.
- Safety: Cr(VI) reagents are toxic and strongly oxidising; quench to Cr(III) (green) before disposal.
Mechanism — Hydrate First, Chromate Ester, β-Elimination (6 Steps)
Mechanistic Checklist (Exam Focus)
- Hydrate-first logic: show gem-diol formation; chromate attacks the hydrate, not the bare carbonyl.
- Chromate ester + β-elimination: depict the chromate ester before the E2-like step that forms the C=O and ejects Cr(VI).
- Cr(VI) reduction: note orange → green colour change as Cr(VI) becomes Cr(III).
- Aldehydes only: ketones do not advance to acids under these aqueous Cr(VI) conditions.
- Formaldehyde oxidises fastest (nearly 100 % hydrate); aryl aldehydes are slower but still proceed.
- Closed-shell pathway: no radicals in the accepted mechanism.
Worked Examples
Benzaldehyde → Benzoic acid (Na₂Cr₂O₇/H₂SO₄)
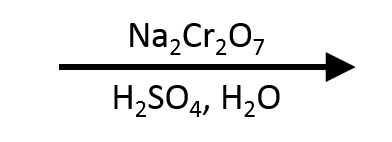
Aromatic aldehydes hydrate more slowly, but chromate ester formation still drives oxidation to benzoic acid; the classic orange → green shift signals Cr(VI) reduction.
Hexanal → Hexanoic acid (K₂Cr₂O₇/H₂SO₄)
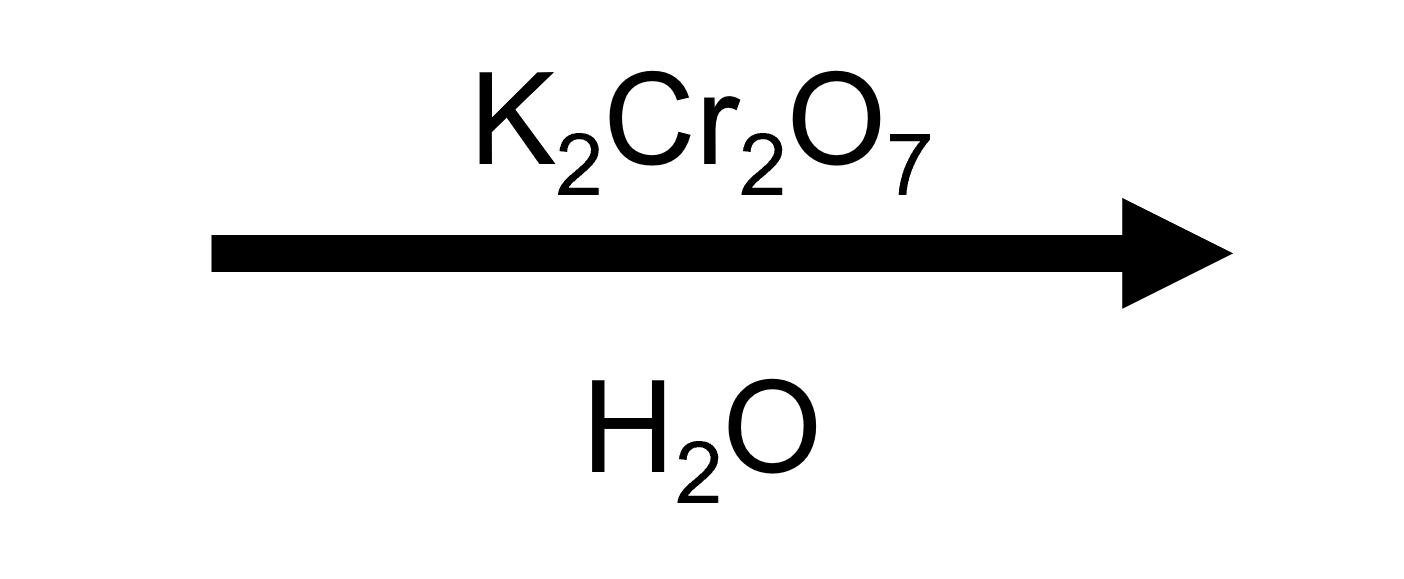
Unbranched aliphatic aldehydes hydrate quickly, so the K₂Cr₂O₇ button delivers quantitative oxidation to the straight-chain carboxylic acid.
Cyclohexanecarboxaldehyde → Cyclohexanecarboxylic acid (CrO₃/H₂SO₄)
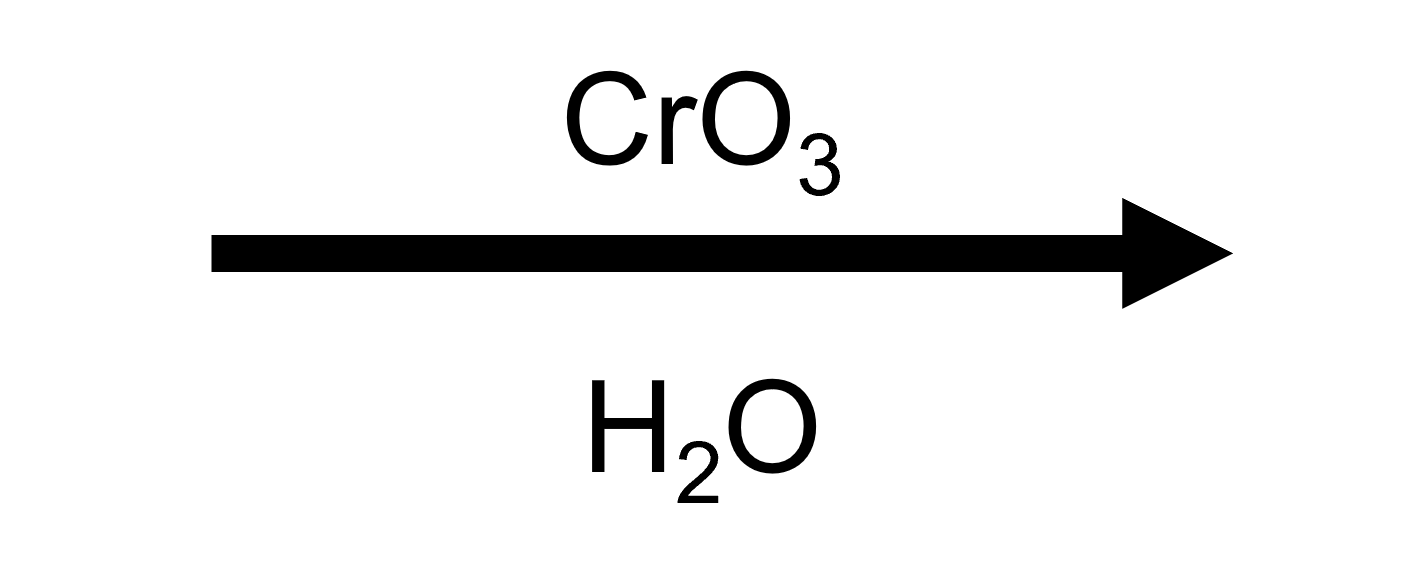
Jones reagent oxidises benzylic and aliphatic aldehydes alike; cyclic aldehydes stay intact because the chromate pathway only modifies the side-chain carbonyl.
Scope & Limitations
- Works best: Aliphatic, benzylic, heteroatom-activated aldehydes; formaldehyde is extremely fast.
- Slower: Aryl aldehydes (hydrate fraction smaller) but still oxidise in Jones/dichromate solutions.
- Not covered: Ketones (do not oxidise to acids here), aldehydes in strictly anhydrous media (hydrate suppressed).
- Functional groups: Oxidises susceptible alcohols/allylic positions in the same pot; protect acid-sensitive groups if needed.
Practical Tips
- Prepare chromate in aqueous acid, then add the aldehyde (often dissolved in acetone for Jones reagent).
- Monitor colour: orange Cr(VI) fades to green Cr(III) as oxidation completes.
- Quench excess Cr(VI) (NaHSO₃ or i-PrOH) before workup; collect chromium waste separately.
- Ensure water is present—hydrate formation is required to reach the acid.
Exam-Style Summary
In water/acid, aldehydes hydrate to gem-diols that form chromate esters. β-Elimination (formally hydride transfer) produces the carboxylic acid and reduces Cr(VI) to Cr(III). Na₂Cr₂O₇, K₂Cr₂O₇, CrO₃ (Jones), and H₂CrO₄ all converge on this pathway; ketones do not over-oxidise under these conditions.
Interactive Toolbox
- Mechanism Solver — Use Mechanism Solver to see each step of the chromate oxidation mechanism along with descriptions of each step!
- Reaction Solver — Quickly find the product of any aldehyde reacted with Na₂Cr₂O₇, K₂Cr₂O₇, CrO₃, or H₂CrO₄!
- IUPAC Namer — Learn the naming ins and outs of aldehyde starting materials and carboxylic acid products.
Related Reading
- Prefer silver(I) conditions? Explore Aldehyde → Carboxylic Acid with Silver (Ag₂O/H₂O) for the Tollens-style oxidation pathway.