Aldehyde/Ketone to Alcohol (NaBH4, MeOH)
Exam Answer
- Reagents
- NaBH4 in MeOH (or EtOH)
- Major outcome
- Aldehyde/ketone to alcohol
- Selectivity
- Typically does not reduce esters/amides in standard exam assumptions
Common traps
- Do not reduce esters/amides with NaBH4 in typical exam problems
- Workup/protonation gives the alcohol
- Stereochemistry depends on substrate face selectivity
NaBH4 vs LiAlH4
| Reagent | Reduces | Notes |
|---|---|---|
| NaBH4 | aldehydes/ketones | mild; alcohol solvents OK |
| LiAlH4 | esters/amides/nitriles plus carbonyls | strong; requires workup |
Sodium borohydride (NaBH4) is a mild, selective hydride donor. In methanol it reduces aldehydes to primary alcohols and ketones to secondary alcohols through polar hydride addition followed by protonation from the solvent. A separate acid workup is not strictly required (though an aqueous wash is routine to hydrolyze borates). Compared with LiAlH4, NaBH4 is safer and more chemoselective: most carboxylic acids, esters, and amides remain untouched under standard MeOH conditions. In α,β-unsaturated carbonyls, NaBH4/MeOH favors 1,2-reduction to the allylic alcohol; adding CeCl3·7H2O (Luche conditions) enhances that 1,2 preference.
Quick Summary
- Reagents/conditions: NaBH4 (1–1.5 equiv per carbonyl) in MeOH, 0 °C -> room temperature. H2 evolution is expected-add portionwise and vent.
- Outcomes: Aldehydes -> primary alcohols; ketones -> secondary alcohols.
- Selectivity vs LiAlH4: NaBH4/MeOH typically leaves carboxylic acids, most esters, and amides untouched at room temperature, while LiAlH4 reduces them aggressively.
- α,β-Unsaturated carbonyls: Default outcome is 1,2-reduction (allylic alcohol). Luche conditions (NaBH4/CeCl3) enhance this bias.
- Stereochemistry: New stereocenters form racemates with achiral NaBH4; diastereoselection follows Felkin–Anh trends in non-chelating solvents.
Mechanism - Hydride Transfer, Solvent Protonation, Product Release (3 Frames)
Mechanistic checklist
- Draw the closed-shell hydride transfer (no radicals/carbocations).
- Show the borate-bound alkoxide in Step 1; only introduce the free alcohol after protonation by MeOH.
- Aldehydes reduce faster than ketones-useful for chemoselective reductions.
- α,β-Unsaturated substrates give allylic alcohols (1,2-reduction); annotate Luche conditions if CeCl3 is added.
- Carboxylic acids/esters/amides normally remain untouched under these conditions.
Worked Examples
Benzaldehyde -> Benzyl alcohol
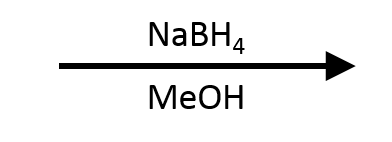
Aldehydes reduce rapidly; benzyl alcohol forms cleanly after hydride addition and in-solvent protonation.
Cyclohexanone -> Cyclohexanol
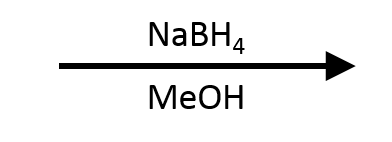
Ketones give secondary alcohols; prochiral reductions produce racemic mixtures under achiral NaBH4 conditions.
Cinnamaldehyde -> Cinnamyl alcohol
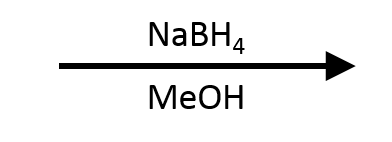
Protical NaBH4 reductions of enals give allylic alcohols (1,2-addition). Adding CeCl3 (Luche) reinforces this selectivity.
Acetophenone -> 1-phenylethanol (racemic)
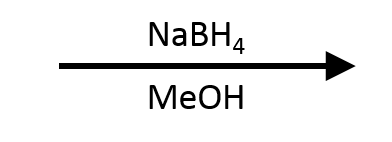
Reduction of a prochiral ketone yields a racemic secondary alcohol unless a chiral reagent/catalyst is used.
Scope & Limitations
- Great: Aliphatic, benzylic, cyclic aldehydes and ketones; enones (1,2 reduction).
- Moderate: Acid chlorides and anhydrides can reduce but are slower; activated esters may react.
- Poor: Carboxylic acids, most esters, and amides generally survive NaBH4/MeOH unless a Lewis acid or elevated temperature is used.
- Functional groups: Allylic halides may slowly reduce; otherwise, NaBH4/MeOH is tolerant of many heteroatoms.
- Solvent tweak: Switching to EtOH or EtOH/H2O slows NaBH4 decomposition; i-PrOH/protic–aprotic mixtures provide further control when needed.
Edge Cases & Exam Traps
- Do not show the alcohol before protonation-depict the borate-bound alkoxide first.
- Assuming NaBH4/MeOH reduces esters/acids is incorrect (unless activated or heated).
- For enones, expect 1,2-products. If a question expects 1,4-reduction, look for metal-catalysed or dissolving-metal conditions.
- Stoichiometry: NaBH4 theoretically delivers four hydrides, but MeOH slowly destroys the reagent-use slight excess in practice.
- Luche conditions (CeCl3) often appear in exam questions; note why they enhance 1,2-selectivity.
Practical Tips
- Charge NaBH4 portionwise at 0–5 °C; allow to warm to room temperature for completion. Vent to release H2 safely.
- Quench by cautiously adding water or saturated NH4Cl; a mild acid rinse removes borate residues.
- For chemoselective aldehyde reduction in the presence of ketones, monitor reaction closely and quench early.
- When sensitive to borate residues, follow with NaOH/H2O2 or simply filter through a short pad of celite after aqueous workup.
- For enones requiring higher 1,2-selectivity, add CeCl3·7H2O (Luche) while keeping the same hydride/protonation steps.
Exam-Style Summary
NaBH4 in MeOH delivers hydride to the carbonyl carbon, forming a borate-bound alkoxide that the solvent protonates to the alcohol. See the stronger LiAlH4 protocol for broader reductions: LiAlH4/H3O+ carbonyl reduction. Aldehydes reduce faster than ketones; α,β-unsaturated carbonyls yield allylic alcohols (especially under Luche conditions). NaBH4/MeOH is milder and more chemoselective than LiAlH4, leaving most esters, acids, and amides untouched at room temperature.
Interactive Toolbox
- Mechanism Solver - Visualize hydride transfer and solvent protonation; toggle the Luche overlay for enones.
- Reaction Solver - Compare NaBH4/MeOH selectivity against LiAlH4 and flag non-reducible functional groups.
- IUPAC Namer - Confirm the names of the primary/secondary alcohol products produced in these reductions.