Alkane Reactions: Radical Halogenation with X₂/hν
Alkanes react with molecular halogens (Br₂ or Cl₂) under UV light or heat to form alkyl halides through a free-radical chain mechanism. This transformation replaces a C–H bond with a C–X bond and is one of the few ways to functionalize otherwise unreactive alkanes.
Quick Summary
- Reagents/conditions: X₂ (Br₂ or Cl₂) with light (hν) or heat (Δ)
- Outcome: Replaces one C–H bond with C–X; forms HX as byproduct
- Mechanism: Free-radical chain (initiation → propagation → termination)
- Selectivity: Bromination is highly selective for 3° > 2° > 1° C–H; chlorination is less selective
- Stereochemistry: Racemic mixture if a new stereocenter forms (radical intermediates are planar/sp²)
- Common pitfalls: Predicting wrong major product by ignoring selectivity; forgetting that multiple substitution can occur with excess halogen
Mechanism (4 Steps)
The radical halogenation mechanism consists of three phases: initiation, propagation, and termination.
Step 1 — Initiation: Homolytic Cleavage of X₂
UV light (hν) or heat provides enough energy to break the weak X–X bond homolytically, generating two halogen radicals (X·).
Br₂: Bond dissociation energy ≈ 192 kJ/mol Cl₂: Bond dissociation energy ≈ 243 kJ/mol
Each atom retains one electron from the shared pair, shown by fishhook (single-barbed) arrows.
Step 2 — Propagation: Hydrogen Abstraction
A halogen radical abstracts a hydrogen atom from the alkane, forming H–X and a carbon-centered radical. This step is the rate-determining step and explains selectivity differences.
The stability of the carbon radical intermediate determines which hydrogen is most easily abstracted:
- 3° radical (most stable) — three alkyl groups stabilize by hyperconjugation
- 2° radical — two alkyl groups
- 1° radical (least stable) — one alkyl group
Step 3 — Propagation: Halogen Transfer
The carbon radical attacks another X₂ molecule, abstracting one halogen atom and regenerating a halogen radical to continue the chain.
Step 4 — Product Formation
The final alkyl halide product forms, and the regenerated X· radical continues propagating the chain until termination events occur (radical–radical coupling).
Worked Examples
Selectivity: Bromination vs Chlorination
The key exam concept is understanding why bromination and chlorination give different product distributions.
Bromination (Br₂/hν) — Highly Selective
Relative reactivity for H-abstraction:
- 1° H : 2° H : 3° H ≈ 1 : 82 : 1600
Bromination is endothermic in the H-abstraction step, so it has a late, product-like transition state. The stability of the forming radical strongly influences the rate, making bromination highly selective for tertiary positions.
Practical result: If a 3° C–H is present, bromination occurs almost exclusively there.
Chlorination (Cl₂/hν) — Less Selective
Relative reactivity for H-abstraction:
- 1° H : 2° H : 3° H ≈ 1 : 3.8 : 5
Chlorination is exothermic in the H-abstraction step, so it has an early, reactant-like transition state. The energy difference between radical types matters less, making chlorination relatively non-selective.
Practical result: Product mixtures are common; statistical factors (number of equivalent H atoms) often dominate.
Predicting the Major Product
To predict the major product on an exam:
- Identify all unique C–H types (1°, 2°, 3°)
- Count hydrogens at each position
- Apply relative reactivity factors
- Calculate weighted scores: (# of H) × (relative reactivity)
- The position with the highest score gives the major product
Example: 2-Methylbutane + Br₂/hν
- 3° H: 1 hydrogen × 1600 = 1600
- 2° H: 2 hydrogens × 82 = 164
- 1° H (–CH₃): 3 hydrogens × 1 = 3
- 1° H (–CH₂CH₃): 3 hydrogens × 1 = 3
Major product: 2-bromo-2-methylbutane (substitution at the 3° carbon)
Worked Examples
Propane + Cl₂/hν (mixture)
Chlorination gives both 1-chloropropane and 2-chloropropane; the 2° site is favored but not exclusive.
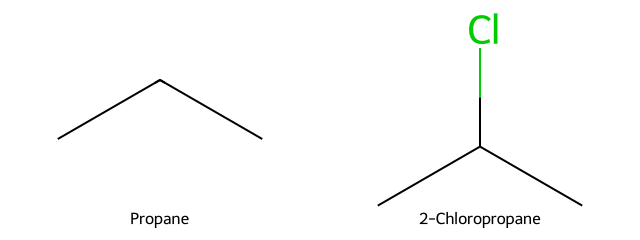
Isobutane (2-methylpropane) + Br₂/hν (highly selective)
One 3° H vs nine 1° H: bromination overwhelmingly gives tert-butyl bromide; the 1° product is trace.
Cyclohexane + Cl₂/hν (symmetric case)
All C–H bonds are equivalent, so monochlorination yields a single constitutional product.
Practical Tips & Pitfalls
- Always identify 3° carbons first — if present with bromination, that's almost certainly the major product
- Don't confuse with ionic mechanisms — radical mechanisms use fishhook arrows (one electron), not full curved arrows (two electrons)
- Watch for symmetry — if all C–H bonds are equivalent (e.g., neopentane, cyclohexane), there's only one possible monohalogenated product
- Multiple substitution — with excess X₂, polyhalogenation can occur; exam questions usually specify "monobromination" or "monochlorination"
Exam-Style Summary
Radical halogenation of alkanes converts C–H to C–X through a chain mechanism initiated by light or heat. The key to exam success is recognizing that bromination is selective (favors 3° >> 2° > 1°) while chlorination is not (gives mixtures based on statistics and modest selectivity). When asked for the major product of monobromination, look for the most substituted C–H and predict substitution there. If a new stereocenter forms, expect a racemic mixture because the radical intermediate is sp²-hybridized (planar).
Common exam traps:
- Forgetting to account for the number of equivalent hydrogens
- Drawing ionic mechanisms instead of radical mechanisms
- Assuming chlorination is as selective as bromination
- Forgetting that radical intermediates are achiral (planar)
Interactive Toolbox
Tap the Br₂/hν button in Mechanism Solver to replay the full radical bromination sequence with overlays.

Tap the Cl₂/hν button in Mechanism Solver to see radical chlorination with the less-selective pathway.
- Mechanism Solver — watch every step of the radical chain mechanism with electron-pushing arrows.
- Reaction Solver — draw any alkane and see the predicted major product of halogenation.
- IUPAC Namer — name the alkyl halide products correctly.
Related Reading
- Allylic Bromination with NBS — selective bromination at allylic positions
- SN2 Substitution — what to do with your alkyl halide product