Alkyl Halide Reactions: Ester Formation using Carboxylate Ions
Alkyl halides react with sodium carboxylate ions (RCO2Na), such as sodium acetate (CH3COONa), to form esters through an SN2 mechanism:
Ester Formation Reaction:
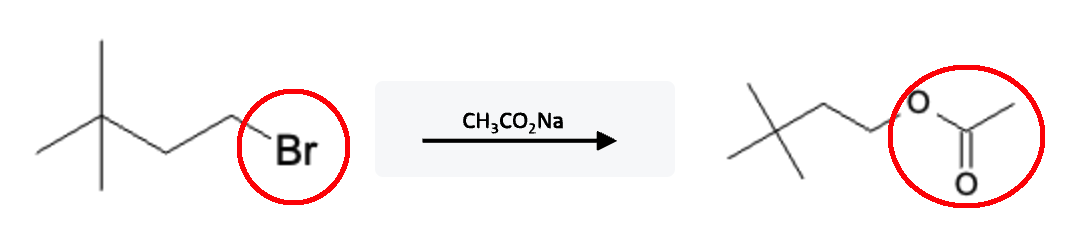
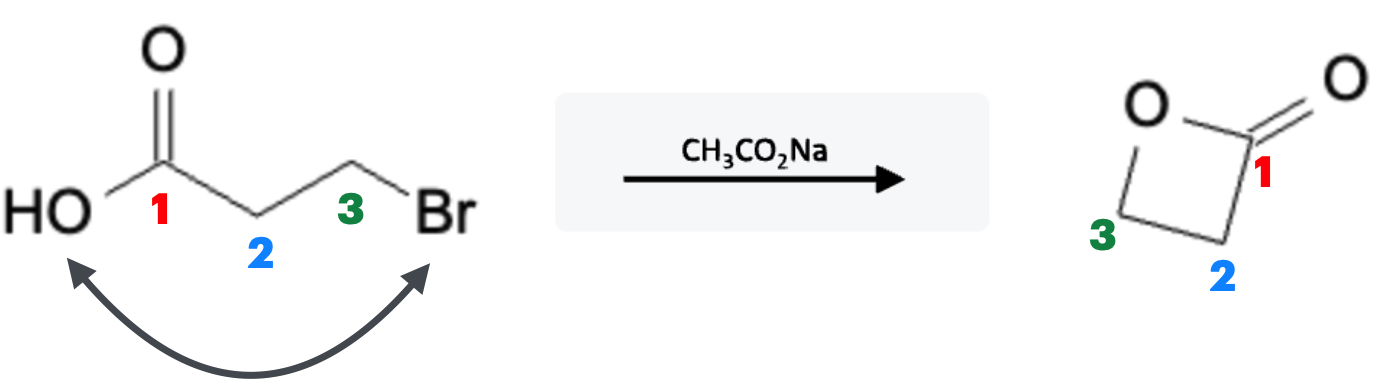
This reaction proceeds through an SN2 reaction, where the carboxylate ion is added at the same time the halide is removed in one concerted step. If the starting reactant molecule has a carboxylate group already attached, an intramolecular reaction can occur thereby forming an ester ring structure:
Ester Ring Structure Reaction:
The reaction mechanism is depicted below using sodium acetate (CH3COONa), but it would be the same using any carboxylate ion; just add the extra carbons to the final product:
Mechanism
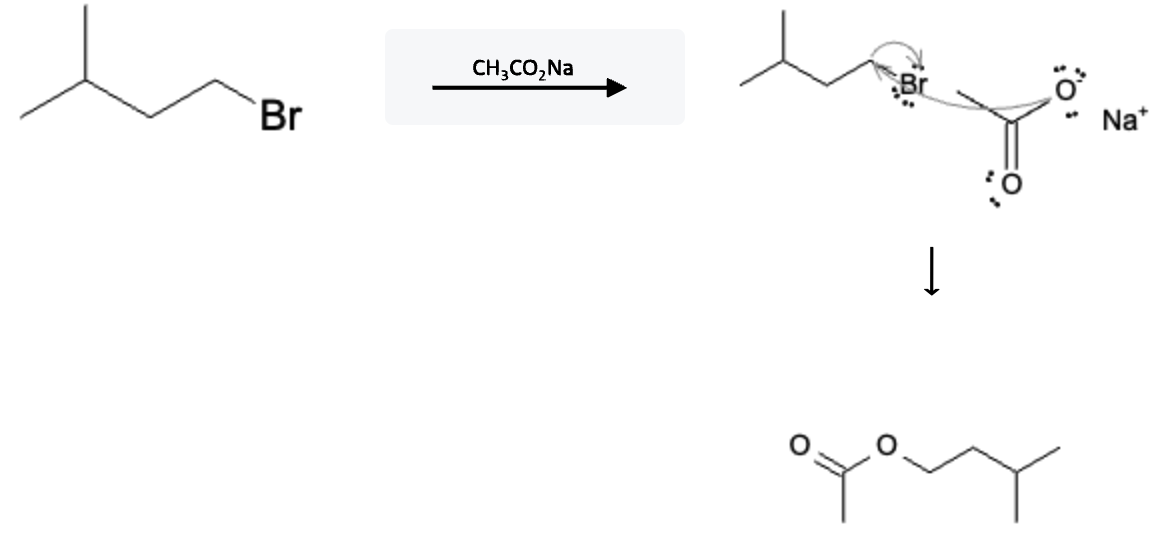
One of the hallmarks of SN2 is that they occur in 1 step! The free electrons from the negatively charged oxygen atom on the CH3COO- molecule attack the carbon where the halide is attached, releasing the halide and adding the acetate moiety.
To learn more about SN2 and E2 reactions and how to distinguish them, be sure to check out our other Lessons!
Practice this reaction using our Reaction Solver!