Alkyl Halide Reactions: SN2 Substitution Reactions
Alkyl halides undergo SN2 substitution reactions when exposed to strong bases (NaOH, NaOMe, NaOEt, etc…). This is in contrast to the E2 elimination reactions that almost exclusively occur when the reaction is run with heat (Δ).
SN2 Subsitution Reactions
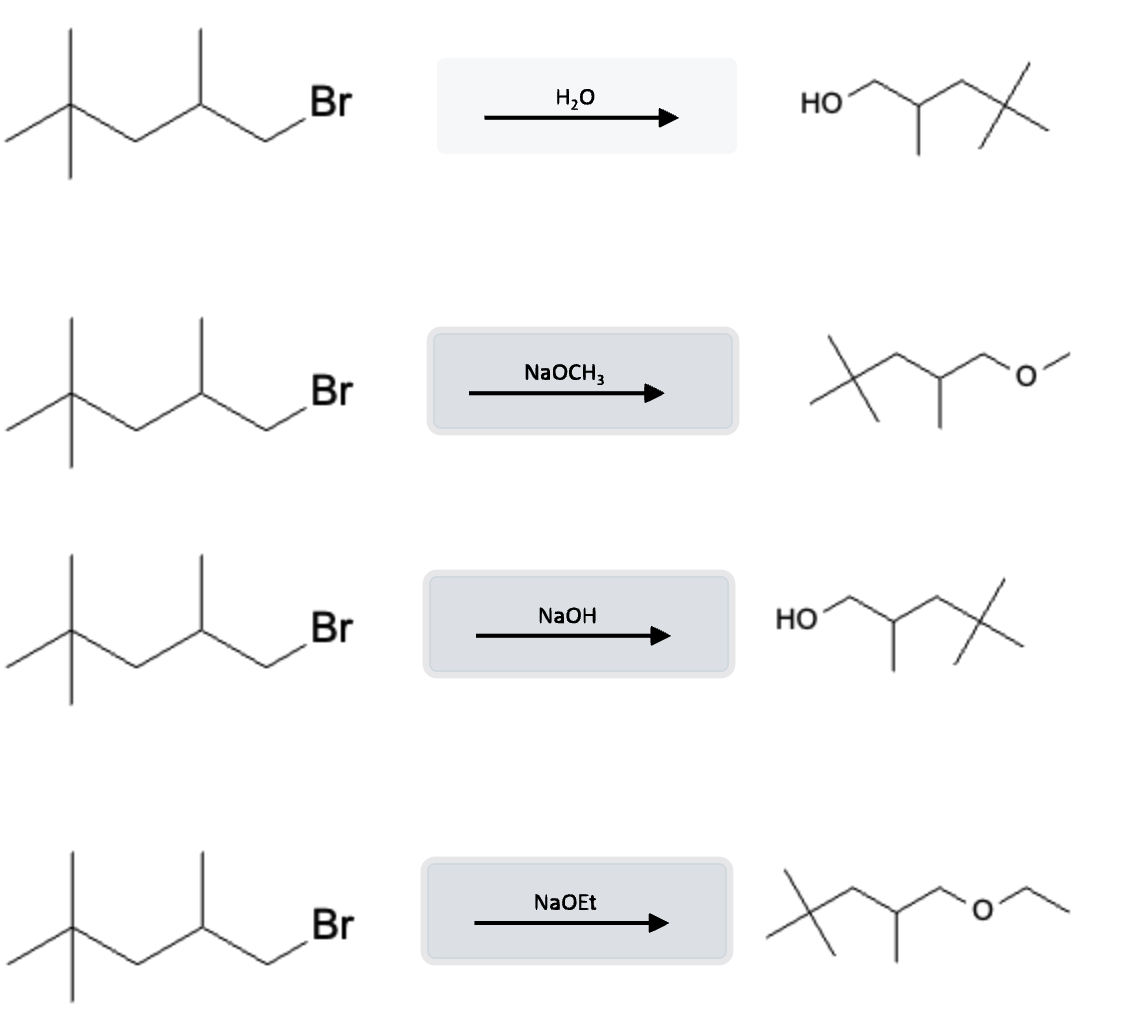
This is in contrast to almost the same reagents that participate in E2 reactions, but those are executed under hot conditions.
E2 Eliminations occur under Heat Conditions
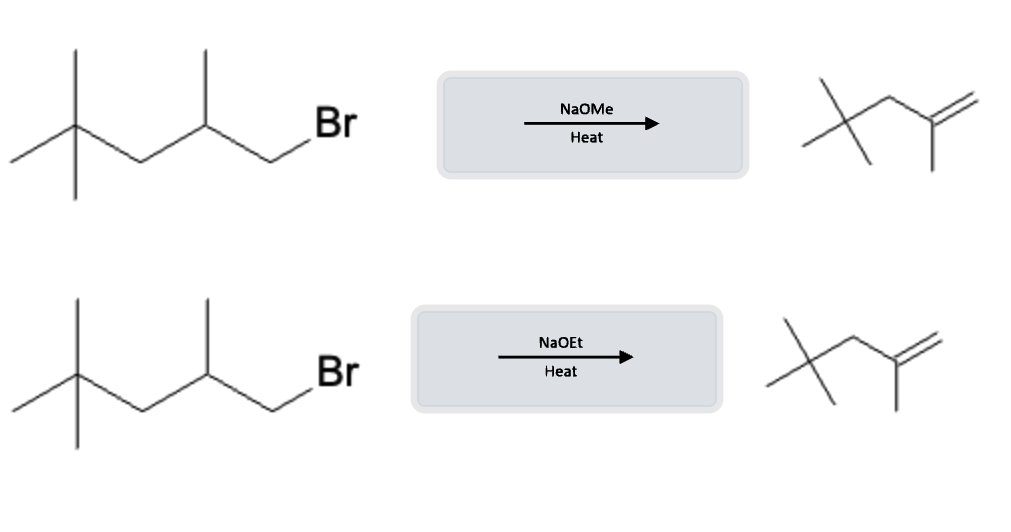
A substitution is more likely to occur with a primary alkyl halide whereas elimination reactions are more likely to occur with secondary and tertiary alkyl halides. Heat can be applied to cause the SN2 substitution to become an E2 elimination.
Mechanism
The reaction mechanism is depicted below using sodium methoxide (NaOMe) however, it would proceed the same way using the other sterically free strong bases:
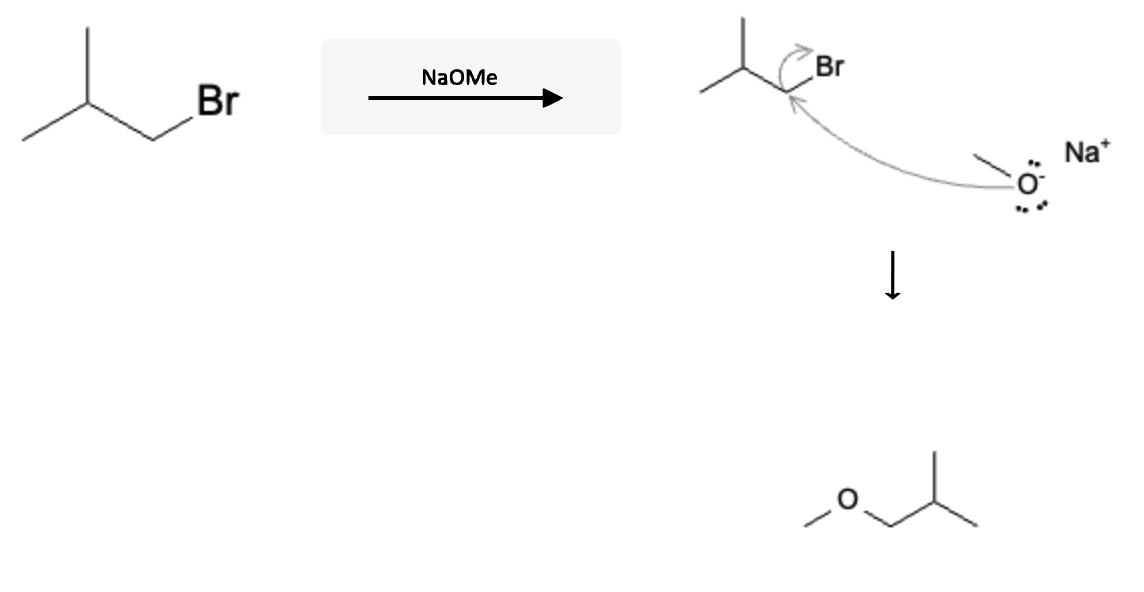
One of the hallmarks of SN2 and E2 reactions is that they occur in 1 step! The free electrons from the negatively charged oxygen atom on the NaOMe molecule attack the carbon atom, releasing the halide (Br in this case).
To learn more about SN2 and E2 reactions and how to distinguish them, be sure to check out our Lessons!
Practice this reaction using our Reaction Solver!