Alkyne Reactions: Acetylide Formation & Alkylation (NaNH₂; R–X)
Terminal alkynes (pKₐ ≈ 25) are acidic enough that sodium amide (NaNH₂; conjugate acid NH₃, pKₐ ≈ 38) deprotonates them completely. The resulting alkynyl (acetylide) anion is a potent, linear nucleophile/base that performs SN2 on methyl, primary, allylic, or benzylic halides/tosylates, forging new C–C σ bonds. With acetylene itself, two rounds of deprotonation/alkylation deliver unsymmetrical internal alkynes. Selectivity is all about the electrophile: hindered (2°/3°) halides eject via E2, while vinyl/aryl halides are inert to SN2.
Key Emphasis (Learning Pivots)
- pKₐ logic drives deprotonation. NH₂⁻ (conjugate acid NH₃, pKₐ ≈ 38) comfortably removes the sp–C–H (pKₐ ≈ 25) of terminal alkynes, giving a metalated acetylide.
- SN2 only works on unhindered halides. Methyl, primary, allylic, and benzylic halides/tosylates undergo clean substitution; secondary/tertiary electrophiles give E2 instead.
- Chain extension: Acetylene can be alkylated twice (R¹ then R²) to furnish unsymmetrical internal alkynes.
- Protic incompatibilities: Any –OH, –NH, –CO₂H, or water instantly quenches the acetylide.
- Vinyl/aryl halides are SN2-inert. Use cross-coupling (e.g., Sonogashira) if you must functionalize those carbons.
Quick Summary
- Stage 1 (base): Terminal alkyne + NaNH₂ (liquid NH₃, THF, DMSO) → acetylide + NH₃.
- Stage 2 (alkylation): Acetylide + R–X (I, Br, OTs, OMs; methyl/primary/allyl/benzylic) → SN2 substitution with inversion at the electrophilic carbon.
- Optional Stage: Repeat Stage 1 + Stage 2 when starting from acetylene to install a second R group.
- Outcome: C–C σ-bond construction; stereoinversion at the electrophilic carbon (if chiral).
- Pitfalls: Secondary/tertiary halides → E2 alkenes; vinyl/aryl halides → no SN2; propargyl halides may give SN2′ (allene) side-products.
Mechanism — Three Steps (with optional repetition)
This optional frame now renders the R placeholder directly (not the earlier asterisk), reinforcing that every addition hands the same R over to the next bond.
Mechanistic Checklist (Exam Focus)
- Terminal alkyne required (must possess ≡C–H).
- SN2 scope limited to methyl/primary/allyl/benzylic; secondary/tertiary halides undergo E2.
- Inversion occurs at the electrophilic carbon (if stereogenic).
- Protic functionalities or water quench the acetylide instantly.
- Vinyl/aryl halides require cross-coupling (no backside approach).
- Propargyl electrophiles can give SN2′ (allenes) — mention when relevant.
Worked Examples
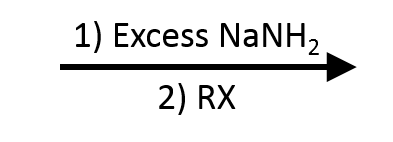
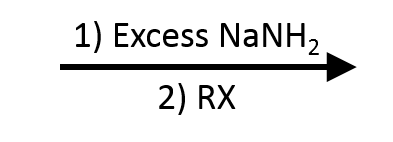
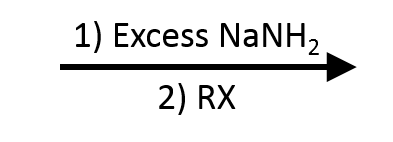
Scope & Limitations
- Best electrophiles: Methyl, primary (esp. allylic/benzylic) halides/tosylates.
- Borderline: Neopentyl-type primary halides (sterically slowed); propargyl halides (possible SN2′ → allenes).
- Poor: Secondary halides (E2 competes), tertiary halides (E2 only), vinyl/aryl halides (no SN2).
- Functional groups: Protect or avoid protic sites that quench acetylide.
- Solvent: Liquid NH₃ for classic protocols; THF/DMSO/DMF for convenience; all must be anhydrous/inert.
Practical Tips & Pitfalls
- Generate the acetylide fully (monitor gas evolution in NaNH₂/NH₃ or NaH/THF protocols) before adding R–X.
- Add alkyl halide slowly to maintain excess acetylide and minimize elimination.
- Prefer I > Br ≫ Cl; tosylates/mesylates are acceptable.
- Keep temperatures low for deprotonation; warm gently for SN2 as needed.
- If E2 dominates, switch to a less hindered electrophile or use milder bases (e.g., NaH) with polar aprotic solvent.
Exam-Style Summary
Terminal alkyne + NaNH₂ → acetylide; acetylide + methyl/primary/allyl/benzylic R–X → SN2 coupling (inversion). Secondary/tertiary halides give E2; vinyl/aryl halides do not undergo SN2. Acetylene can be dialkylated by repeating the sequence.
Interactive Toolbox
Hit the nanh2_rx.png reagent button to launch the Mechanism Solver and replay the acetylide formation + alkylation frames (including the optional repeat from acetylene).
- Mechanism Solver — Animate NaNH₂ deprotonation → SN2 addition (and the optional second alkylation) to reinforce the closed-shell arrow practice.
- Reaction Solver — Compare substitution vs elimination for methyl/primary versus secondary/tertiary halides + terminal alkynes.
- IUPAC Namer — Caption both the starting alkyne and the alkynes produced in the worked examples (no SMILES shown to readers).
FAQ
- Why does NaNH₂ work while NaOH doesn’t? NH₂⁻ has a much stronger conjugate acid, so the equilibrium strongly favors acetylide formation; HO⁻ (pKₐ ≈ 15.7) cannot deprotonate the ≡C–H effectively.
- Can I use secondary halides? Not productively—expect β-elimination to alkenes; pick a less hindered halide.
- Do vinyl/aryl halides participate? No, backside approach is blocked; use Pd-catalyzed couplings instead.
- How do I avoid SN2′ with propargyl halides? Choose electrophiles that lack adjacent π-systems or be prepared to draw both propargyl and allenyl products.
- Is a workup required? Typically NH₄Cl(aq)/H₂O to neutralize excess base and dissolve inorganic salts before isolation.