Alkyne Reactions: Alkyne Halogenation using Br2/Cl2 and CCl4
An alkyne reacts with Br₂ or Cl₂ in an inert, non-nucleophilic solvent (typically CCl₄ or CH₂Cl₂) by electrophilic addition through a halonium intermediate. One equivalent of halogen opens the halonium anti to give the vinyl dihalide (E-1,2-dihaloalkene). With excess halogen, the intermediate alkene undergoes a second anti addition to furnish the 1,1,2,2-tetrahaloalkane. Because the vinyl dihalide is less electron-rich than the parent alkyne, careful control of equivalents, time, and temperature lets you stop at the vinyl dihalide or drive to the tetrahalide. I₂/CCl₄ is sluggish and rarely used; Br₂ or Cl₂ dominate teaching and practice.
Use the toggles in the Interactive Toolbox to experiment with reagent choice and stoichiometry, or send the substrate to the Mechanism Solver for a live halonium walkthrough.
Quick Summary
- Reagents: Br₂/CCl₄ or Cl₂/CCl₄ (room temperature to mild reflux; shield from bright light to suppress radical chains).
- 1 equivalent X₂: Anti addition across the C≡C → predominantly the E-1,2-dihaloalkene (vinyl dihalide).
- Excess X₂: Vinyl dihalide undergoes a second anti addition → 1,1,2,2-tetrahaloalkane.
- Mechanism: Halonium formation → halide backside opening; repeat on the alkene if more X₂ is present.
- Stereochemistry: First addition is anti (E major; internal alkynes may show small E/Z mixtures); second addition gives a saturated tetrahalide, so stereochemistry is moot.
- Solvent role: CCl₄ (or CH₂Cl₂) is inert/non-nucleophilic, preventing halohydrin or haloether formation.
- Comparison: Alkenes generally add X₂ faster than alkynes; plan equivalents/sequence accordingly if both are present.
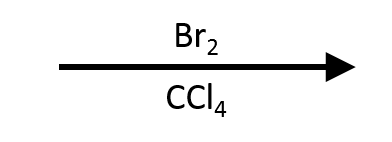
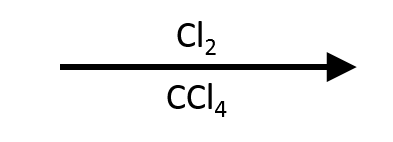
Mechanism (5 Didactic Frames)
Class: Closed-shell electrophilic addition. Two back-to-back halonium formations, each followed by anti opening, funnel the alkyne to the tetrahalide without radicals or vinyl cations.
- Halonium formation on the alkyne: The π bond polarises X₂; X⁺ bridges the C≡C to avoid a vinyl cation.
- Anti opening to the vinyl dihalide: Halide attacks from the backside, delivering the E-1,2-dihaloalkene.
- Second halonium (excess X₂): The vinyl dihalide’s C=C repeats the halonium formation when more halogen is present.
- Second anti opening: Halide opens the new bridge anti, installing the remaining halogen on each carbon.
- Product frame: The 1,1,2,2-tetrahaloalkane captures the net result (mixed sequences place one halogen of each type on every carbon).
Each panel below is a teaching snapshot; in practice the additions flow smoothly under polar control.
Polarisation of X₂ gives the bridged halonium, avoiding a vinyl cation.
↓
Halide attacks from the backside to furnish the E vinyl dihalide.
↓
With additional halogen, the C=C repeats the halonium formation.
↓
Halide opens the bridge anti once more, adding the final X to each carbon.
↓
The reaction ends with the 1,1,2,2-tetrahaloalkane (mixed sequences give one of each halogen per carbon).
Mechanistic Checklist (Exam Focus)
- Always draw a halonium intermediate—no free vinyl cations or radicals in this polar pathway.
- First equivalent executes anti addition → E vinyl dihalide predominates; draw both E/Z if the question expects them.
- Excess X₂ adds again through halonium → 1,1,2,2-tetrahaloalkane (all four C–X bonds).
- Mixed halogen sequences place one of each halogen on every saturated carbon.
- Anhydrous, non-nucleophilic solvent (CCl₄, CH₂Cl₂) keeps the path on-track; water/alcohol diverts to halohydrins/haloethers.
- No rearrangements or hydride shifts—bond-making/breaking stays local to the π system.
Worked Examples
Example A — Symmetric internal alkyne (but-2-yne scaffold)

- Symmetry keeps regiochemistry straightforward; both carbons receive identical substituents.
- The E vinyl dihalide is the kinetic product; the tetrahalide appears only with additional bromine.
Example B — Bulky internal alkyne (tert-alkyl substituent)

- Steric bulk moderates rate but not the anti selectivity; halide attack still approaches from the backside.
- Mixed sequences (e.g., Br₂ then Cl₂) would place one Br and one Cl on each carbon of the saturated product.
Practical Tips & Pitfalls
- Stoichiometry: Monitor by TLC/GC to stop at the vinyl dihalide or push to the tetrahalide.
- Light control: Perform in amber glassware or under reduced light to minimise radical side pathways.
- Solvent choice: Stick with inert solvents (CCl₄, CH₂Cl₂); nucleophiles shift the chemistry toward halohydrins.
- Safety: Br₂/Cl₂ are corrosive oxidants and CCl₄ is toxic—use a hood, gloves, and quench excess halogen with aqueous thiosulfate.
Exam-Style Summary
Alkyne + X₂ (Br₂ or Cl₂) in CCl₄ →
• 1 equiv: Anti addition via halonium → (E)-1,2-dihaloalkene.
• Excess: Second anti addition across the C=C → 1,1,2,2-tetrahaloalkane.
• Mixed halogens: Sequential steps install one halogen of each type on every saturated carbon.
Interactive Toolbox
- Reaction Solver — predict major halogenation products for custom alkynes.
- Mechanism Solver — render the full halonium pathway with adjustable reagents.
- IUPAC Namer — confirm systematic names for vinyl dihalides and tetrahalides.
FAQ / Exam Notes
Why is the addition anti? Backside attack on the halonium bridge enforces anti stereochemistry.
Can I stop at the vinyl dihalide? Yes—use 1 equiv X₂, monitor closely, and limit reaction time.
Does the solvent matter? Absolutely—CCl₄/CH₂Cl₂ are inert; protic solvents give halohydrins instead.
What about I₂/CCl₄? Too sluggish for most alkynes; Br₂ or Cl₂ are the teaching standards.
Are rearrangements possible? No, the pathway is polar and concerted around the π bond.