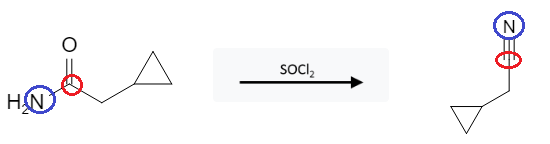
Amide Reactions: Dehydration of Primary Amides to form Nitriles using SOCl2
Primary amides can be dehydrated with agents such as thionyl chloride (SOCl2) or phosphorus pentoxide (P2O5) to form nitriles:
This reaction is pretty straight forward; the double bonded O gets removed and the nitrogen bond goes from a single bond to a triple bond. There aren’t any “trick” reactions with this reagent and functional group.
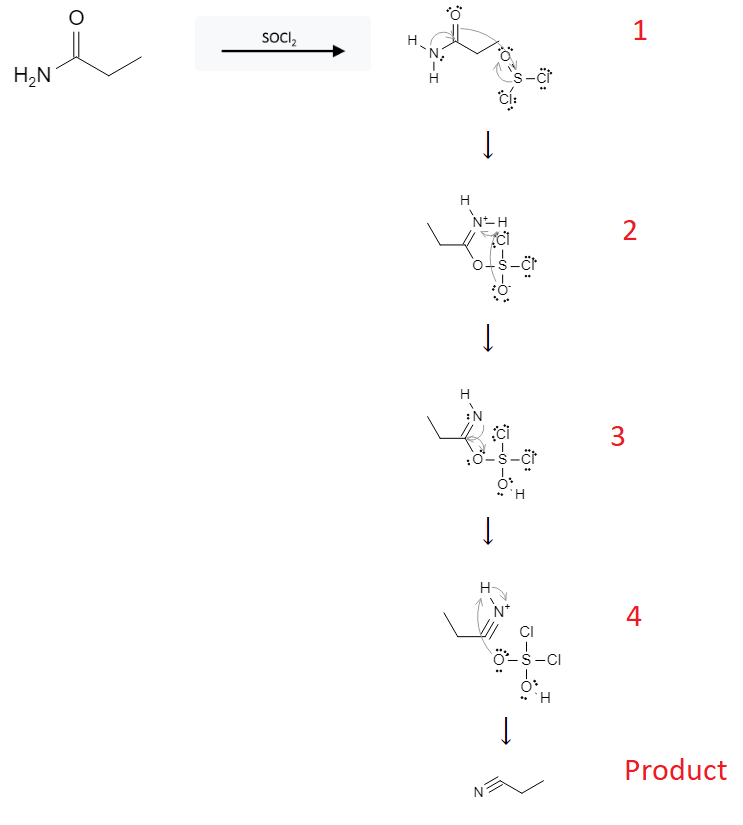
The reaction mechanism is depicted below:
In the first step, the double bond electrons from the oxygen on the amide group attack the sulfur atom on SOCl2, which allows the lone pair electrons on nitrogen to form a double bond with carbon, and forces the double bond on the SOCl2 molecule to break and send the electrons to the SOCl2 oxygen atom..
In the second step, the lone pair electrons from the SOCl2 oxygen atom attack the proton on the nitrogen atom, sending the electrons from that bond to the positively charged nitrogen atom.
In the third step, the lone pair electrons on the nitrogen atom form a triple bond with the carbon atom, allowing the oxygen atom to break its bond and the SO2Cl2 molecule to break away.
In the fourth step, the negatively charged oxygen atom on the SO2Cl2 molecule removes the proton from the nitrogen atom, completing the reaction.