Aromatic Reactions: Friedel-Crafts Alkylation and Acylation
Benzene molecules react with acid chlorides (RCOCl) and alkyl chlorides (RCl), catalyzed by aluminum chloride (AlCl3) to undergo reactions known as the Friedel-Crafts Acylation and Alkylation (respectively). In these reactions, AlCl3 helps catalyze the reaction to add a keto group or alkyl group to the benzene molecule. The location of the addition depends on what functional group(s) is present on the benzene molecule prior to the reaction starting. If the specific position is occupied by another functional group, the reaction will not occur as there is not and available location for the addition to happen:
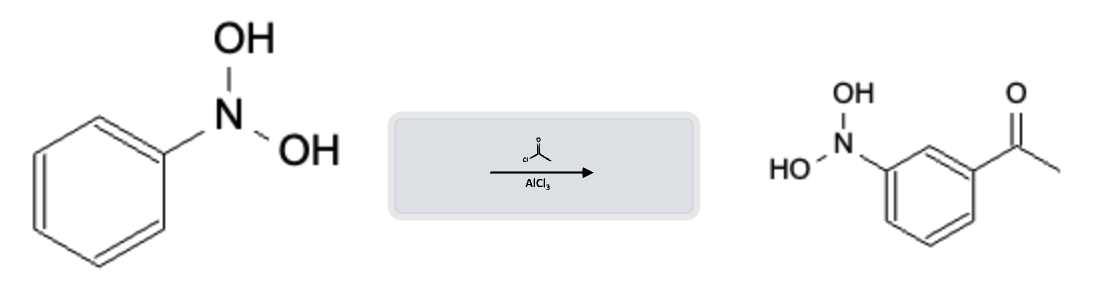
Friedel-Crafts Acylation Electron Withdrawing Group (EWG)
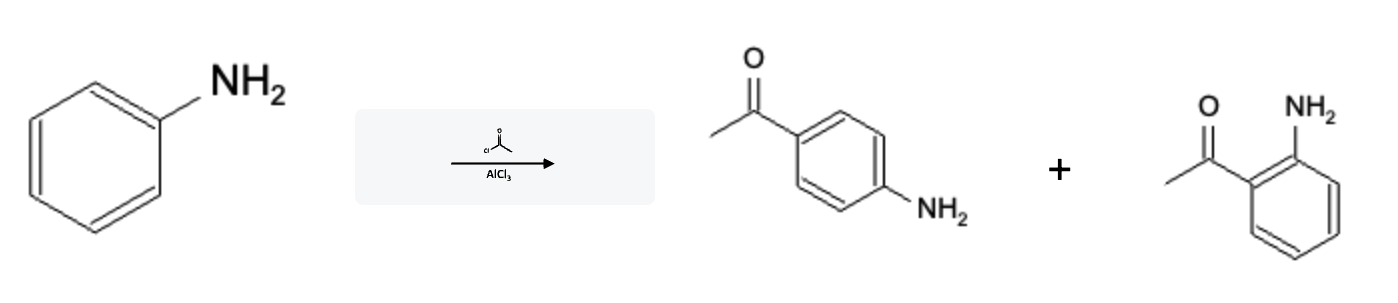
Friedel-Crafts Acylation Electron Donating Group (EDG)
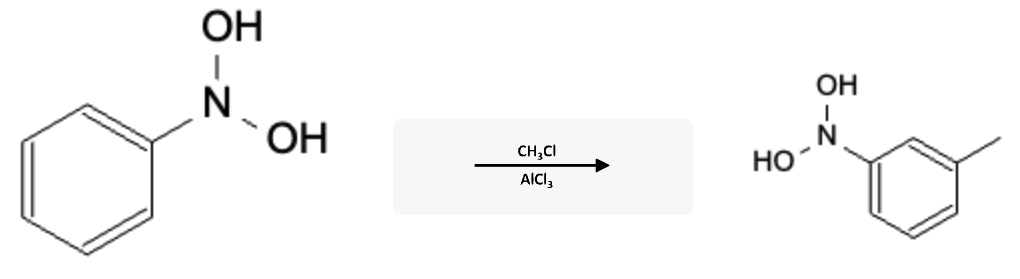
Friedel-Crafts Alkylation Electron Withdrawing Group (EWG)
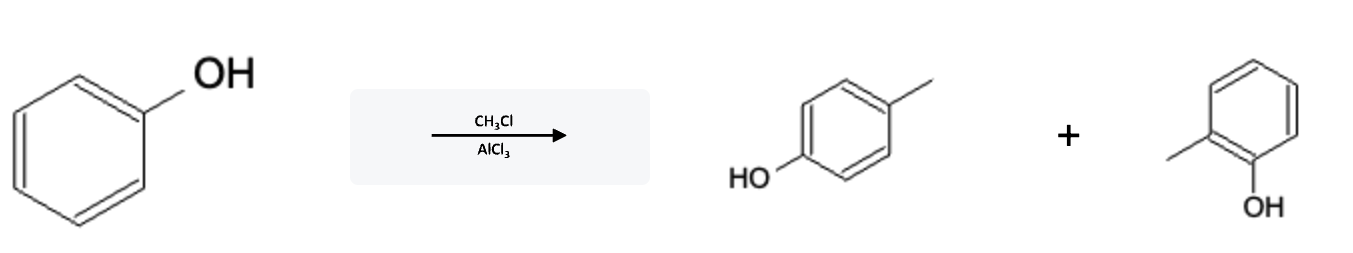
Friedel-Crafts Alkylation Electron Donating Group (EDG)
Mechanism
The reaction mechanism for the alkylation is depicted below, but the acylation occurs in the exact same manner:
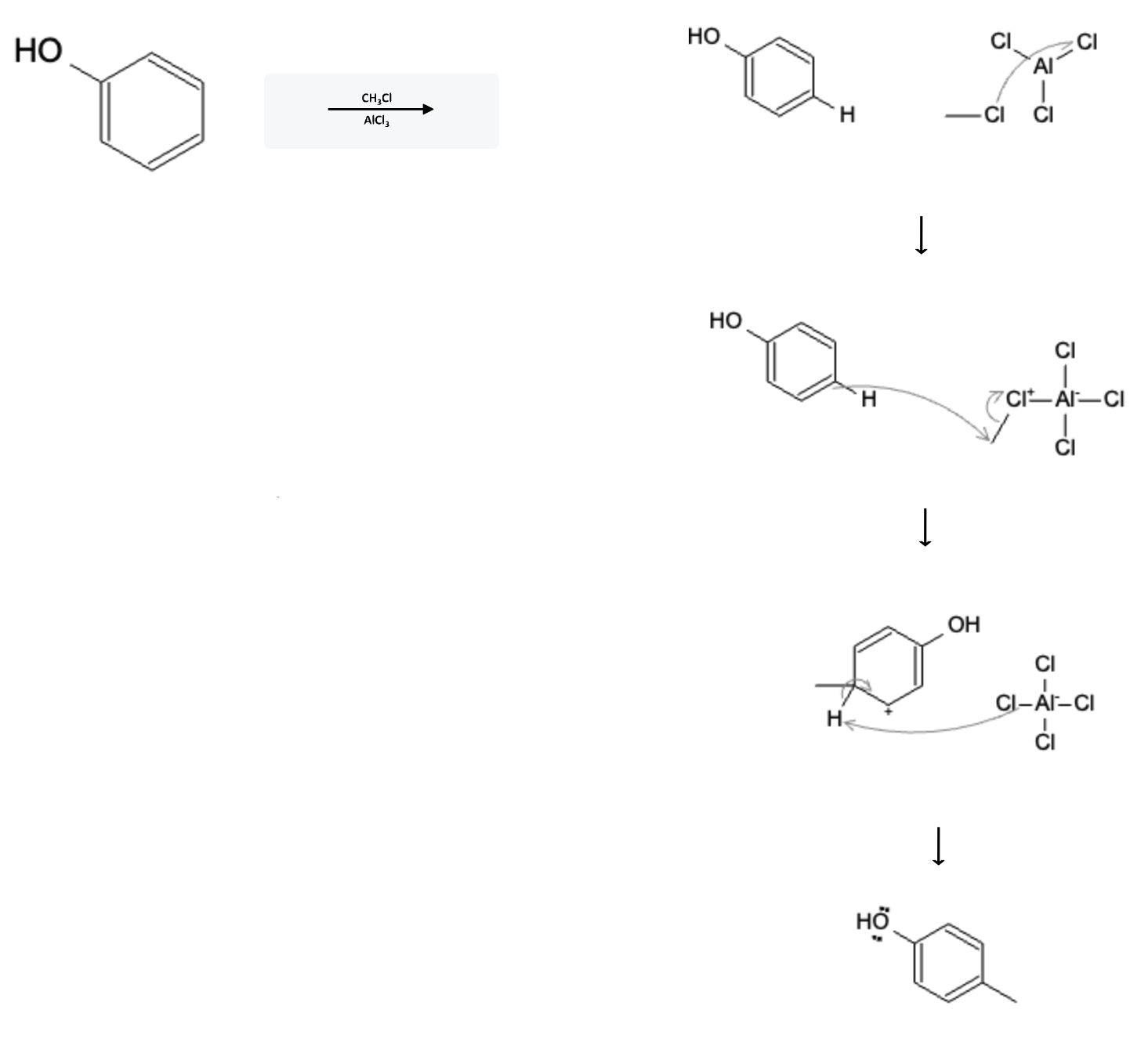
In the first step, the free electrons on the chloride atom from the alkyl halide attack the aluminum atom on the aluminum chloride molecule, priming the molecule for the reaction with benzene.
In the second step, the pi electrons from one of the benzene bonds (in this situation, the pi electrons connected to the para position) react with the alkyl group, thereby breaking the alkyl halide bond resulting in AlCl4.
In the third step, one of the chloride groups from AlCl4 attacks the hydrogen atom attached to the para position, resulting in the chloride group leaving the AlCl4 molecule to form HCl. The electrons from the broken hydrogen bond reform a double bond in the benzene ring, resulting in the final product.
Make sure you learn which groups are considered electron withdrawing and electron donating as professors like to trip students up on this topic. Additionally, check for open positions on the benzene ring; if the para position had been occupied in the mechanism example above, the reaction could proceeded on either ortho position.
Practice this reaction using our Reaction Solver!