Aromatic Reactions: Gatterman-Koch Formylation
The Gattermann–Koch formylation installs a formyl group (–CHO) onto benzene and many activated arenes through electrophilic aromatic substitution (EAS). Passing CO and dry HCl over an arene in the presence of AlCl₃ (Lewis acid) and a CuCl promoter generates a highly reactive formyl electrophile, often depicted as the formylium cation (H–C≡O⁺) complexed to the acids. The arene attacks this electrophile to give a σ-complex, deprotonation restores aromaticity, and aqueous workup liberates the free aryl aldehyde. Like Friedel–Crafts acylation, no carbocation rearrangements occur, and the product –CHO is deactivating/meta-directing, so monoformylation predominates.
Key Emphasis (Teaching Pivots)
- Electrophile identity: CO/HCl under AlCl₃/CuCl forms a formyl (acylium) cation—essentially a Friedel–Crafts acylium with R = H.
- Canonical EAS frames: Electrophile generation → π-attack → σ-complex → deprotonation (rearomatization) → aqueous workup to release Ar–CHO.
- Orientation & deactivation: Existing EDG/EWG groups control the first substitution (EDG → o/p, EWG → meta). The installed –CHO is meta-directing, so monoformylation dominates.
- Feasibility window: Works best on activated/neutral rings. Strong meta directors (–NO₂, –CF₃, –SO₃H) and unprotected amines/phenols shut down the reaction.
- Exam contrasts: Not the same as Vilsmeier–Haack (DMF/POCl₃) or Reimer–Tiemann (phenols, CHCl₃/NaOH). GK specifically uses CO/HCl and Lewis acids.
Quick Summary
- Reagents: CO (g), dry HCl (g), AlCl₃ (Lewis acid), CuCl (promoter), dry CH₂Cl₂/CS₂, 0–25 °C; aqueous workup liberates Ar–CHO.
- Electrophile: Formyl (HCO⁺ / H–C≡O⁺) equivalent generated in situ; no isolable “formyl chloride.”
- Mechanism: CO/HCl activation → π attack (rate-determining) → σ-complex → Cl⁻/AlCl₄⁻-mediated deprotonation → hydrolysis of the Ar–CHO·AlCl₃ adduct.
- Orientation: EDG → o/p (para favored when sterically accessible); EWG → meta. Product –CHO is meta-directing for subsequent EAS.
- Outcome: Monoformylation is typical; rearrangements do not occur because the formyl acylium is resonance-stabilized.
Mechanism — Gattermann–Koch Formylation (8 Frames)
Each frame below uses benzene as the reference arene and mirrors the RDKit Mechanism Solver artwork.
Orientation follows the global EDG/EWG map: activators (alkyl, alkoxy, amides) → ortho/para (para preferred when space allows), deactivators (carbonyls, –NO₂, –CF₃) → meta. The newly installed –CHO is meta-directing for any subsequent EAS, and the Reaction Solver highlights para/ortho products when sterics allow.
Mechanistic Checklist (Exam Focus)
- Always depict CO/HCl + AlCl₃/CuCl generating a formyl electrophile—do not draw “formyl chloride” as a discrete reagent.
- π attack → σ-complex is the rate-determining step.
- Deprotonation requires AlCl₄⁻/Cl⁻, and workup is mandatory to release the free aldehyde.
- Product –CHO groups deactivate and meta-direct, so only one formylation occurs under standard conditions.
- Unprotected –NH₂ / –OH groups coordinate/protonate; protect first (e.g., acetanilide, silyl ethers).
- Strong meta directors (–NO₂, –CF₃, –SO₃H) suppress the reaction—expect “no reaction” outputs.
Worked Examples
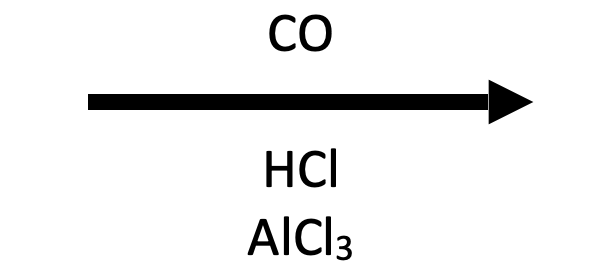
Classic GK conditions (CO/HCl, AlCl₃/CuCl, 0–25 °C) provide benzaldehyde cleanly; the new –CHO meta-directs further EAS.
The methyl group is an ortho/para director; para is favored by sterics, with a minor ortho isomer.
Strong EDG (–OMe) speeds the reaction; para dominates when the reaction is kept cold to avoid demethylation.
Strong meta directors (–NO₂, –SO₃H, –CF₃) render the ring too deactivated for GK conditions—expect “no reaction.”
Scope & Limitations
- Works well: Benzene, alkylbenzenes, anisole/aryl ethers, activated rings with moderate EDG groups.
- Challenging or blocked: Strong meta directors (–NO₂, –CF₃, –SO₃H, multiple carbonyls). Unprotected –NH₂/–OH coordinate to AlCl₃ or are protonated—protect them first (acetanilide, silyl ether).
- Regioselectivity: Use protecting/blocking groups when ortho/para conflicts exist. The installed –CHO meta-directs any follow-up EAS transformations.
- Process constraints: Absolutely anhydrous—glassware, solvent, and salts must be dry; CO/HCl gas handling requires dedicated hardware and safety controls.
- Alternatives: Vilsmeier–Haack (DMF/POCl₃) is often gentler; Reimer–Tiemann targets phenols under basic conditions.
Edge Cases & Exam Traps
- Confusing GK with Vilsmeier–Haack or Reimer–Tiemann. All install –CHO but with very different reagents/conditions.
- Over-formylation “myth”: –CHO deactivates strongly, so a second substitution is rarely observed.
- Amines/phenols must be protected; otherwise the Lewis acid seizes them and the ring doesn’t react.
- CO and HCl are hazardous gases—real labs stress engineering controls; watch for safety prompts in UI/exams.
Practical Tips
- Pre-dry everything (glassware, solvent, AlCl₃, CuCl). Trace water quenches the electrophile.
- Charge AlCl₃/CuCl and solvent first, add the arene, then bubble HCl followed by CO while maintaining 0–25 °C.
- Quench into ice/water to hydrolyze Al complexes; separate the organic phase carefully to recover the aldehyde.
- Slow or stubborn substrates may respond to slightly higher CO pressure or switching to a Vilsmeier protocol.
Exam-Style Summary
CO/HCl + AlCl₃/CuCl generates a formyl electrophile (HCO⁺). Benzene performs π attack → σ-complex → deprotonation, and aqueous workup liberates Ar–CHO. No rearrangements occur, and the new –CHO meta-directs further EAS, so monoformylation is the norm.
Interactive Toolbox
- Use Mechanism Solver to watch CO/HCl activation, acyl-chloride ligation, formylium attack, and workup play out with narrated arrows.
- Use Reaction Solver to predict ortho/para/meta outcomes, test blocking groups, and confirm when strong meta directors halt GK formylation.
- Use IUPAC Namer to drill benzaldehyde derivative names produced by CO/HCl/AlCl₃/CuCl conditions.